Abstract
Purpose
Although current guidelines recommend continuing the same antithrombotic strategy regardless of rhythm control after radiofrequency catheter ablation (RFCA) of atrial fibrillation (AF), anticoagulation has a risk of major bleeding. We evaluated the safety of switching warfarin to aspirin in patients with successful AF ablation.
Materials and Methods
Among 721 patients who underwent RFCA of AF, 608 patients (age, 57.3±10.9 years; 77.0% male, 75.5% paroxysmal AF) who had no evidence of AF recurrence at 3 months post-RFCA were included. We compared the thromboembolic and hemorrhagic events in patients for whom warfarin was switched to aspirin (ASA group; n=296) and patients who were kept on warfarin therapy (W group; n=312).
Results
There were no significant differences in CHA2DS2-VASc or HAS-BLED scores between the groups. In 30 patients in the ASA group and 37 patients in W group, AF recurred and warfarin was restarted or maintained during the 18.0±12.2 months of follow-up. There were no significant differences in thromboembolic (0.3% vs. 1.0%, p=0.342) and major bleeding incidences (0.7% vs. 0.6%, p=0.958) between ASA and W groups during the follow-up period. In the 259 patients with a CHA2DS2-VASc score ≥2, there were no significant differences in thromboembolism (0.8% and 2.2%, p=0.380) or major bleeding incidences (0.8% and 1.4%, p=0.640) between ASA and W groups.
Atrial fibrillation (AF) is significantly associated with the risk of strokes,1,2 and given this association, antithrombotic therapy is essential in the management of AF. CHADS2 or CHA2DS2-VASc scores have been utilized for risk stratification of ischemic stroke in patients with AF,3,4 and anticoagulation is recommended for the patients with CHADS2 score ≥1 or CHA2DS2-VASc score ≥1.5,6 Radiofrequency catheter ablation (RFCA) has been known to be as effective as or more effective than antiarrhythmic drugs (AAD) at controlling rhythm in AF patients.7,8,9 According to the 2012 HRS/EHRA/ECAS Expert Consensus Statement, AAD-resistant symptomatic paroxysmal AF (PAF) and persistent AF (PeAF) are class I and IIa indications for RFCA, respectively.10 However, the same guidelines recommend that anticoagulation be continued after RFCA of AF based on CHADS2 or CHA2DS2-VASc score.10 Although warfarin is effective for the prevention of stroke in patients with AF, using warfarin is difficult given the various dose-effect relationships as well as potential drug-drug or drug-food interactions. Moreover, warfarin introduces an annual risk of major bleeding of 3.1-3.7%.11,12,13 Of the various bleeding risk stratification schemas, the HAS-BLED score is recommended by the current guidelines for prediction of bleeding risk in patients who take warfarin due to AF,5,14 and special attention should be paid to patients with high HAS-BLED score. Given the risks and benefits of warfarin, controversies remain regarding long-term use of warfarin after successful RFCA of AF. Therefore, we hypothesized that switching warfarin to aspirin (ASA group) may have an equivalent efficacy and safety in the prevention of ischemic stroke, compared with continuous warfarin administration (W group) in patients with no evidence of AF recurrence after RFCA during standard rhythm monitoring.
The study protocol was approved by the Institutional Review Board of Severance Cardiovascular Hospital, Yonsei University Health System and Asan Medical Center, and adhered to the Declaration of Helsinki. This study included 721 patients who were prospectively registered in a database of AF catheter ablation between March 2009 and December 2011 in two centers. Clinical outcomes, thromboembolic events, and hemorrhagic complications were compared between the ASA group and the W group in the registry database. Among these 721 patients, there were 608 patients (age, 57.3±10.9 years; 77.0%, male; 75.5%, PAF; 24.5%, PeAF) who had no evidence of AF recurrence on Holter monitoring 3 months after RFCA in this study. All patients provided written informed consent, and all were AAD-resistant or intolerant patients. The exclusion criteria were as follows: 1) permanent AF refractory to electrical cardioversion; 2) left atrium (LA) size >55 mm as measured by echocardiogram; 3) critical coronary artery stenosis (>75% of luminal diameter); 4) severe rheumatic mitral valvular disease; and 5) prior RFCA of AF. Three-dimensional (3D) spiral computerized tomography (CT) scans (64 Channel, Light Speed Volume CT, Philips, Brilliance 63, the Netherlands) were performed to visually define pulmonary vein (PV) anatomy. The presence of an LA thrombus was excluded by transesophageal echocardiography. All AADs were discontinued for a period corresponding to at least five half-lives. In total, 141 patients (19.6%) were taking amiodarone, which was discontinued for at least four weeks prior to the procedure. Anticoagulation therapy was maintained before RFCA for all patients.
Intracardiac electrograms were recorded using the Prucka CardioLab™ electrophysiology system (General Electric Health Care System Inc., Milwaukee, WI, USA) or Workmate
(St. Jude Medical Inc., Minnetonka, MN, USA). RFCA was performed in all patients using 3D electroanatomical
mapping (NavX; St. Jude Medical Inc., Minnetonka,
MN, USA or Carto; Johnson & Johnson Inc., Diamond Bar, CA, USA) merged with 3D spiral CT. We used an open irrigated-tip catheter (Celsius or Thermocool, Johnson & Johnson Inc.; Diamond Bar, CA, USA; irrigation flow rate 20 to 30 mL/min; 25-35 W; 47℃) to deliver radiofrequency
energy for ablation (Stockert generator, Biosense Webster Inc.; Diamond Bar, CA, USA). All patients initially underwent circumferential PV isolation (CPVI) and bi-directional
block of the cavotricuspid isthmus. For the patients with PAF, we added linear ablations and complex fractionated
atrial electrogram (CFAE) ablation guided by 3D-CFAE-CL map15 in a stepwise approach in patients without AF termination
during RFCA. For PeAF, we conducted CPVI, cavotricuspid isthmus block, roof line, posterior inferior line, and anterior line16 as a routine lesion set, and CFAE ablation was added based on the operator's decision. If AF persisted beyond the aforementioned ablation protocols for PAF or PeAF, we stopped the procedure after internal cardioversion. The endpoint of our procedure was no immediate recurrence of AF after cardioversion with isoproterenol infusion (5-20 µg/min). If there were non-PV foci under isoproterenol, we ablated them all. During RFCA, activated clotting time was maintained between 350 to 400 seconds.
All patients were followed up at the outpatient clinic after 1, 3, 6, 9, and 12 months post-RFCA and then every 6 months. Electrocardiography (ECG) was performed at every visit and whenever the patients complained of palpitation. A Holter monitoring (24- or 48-hour) and/or an event recorder were performed at 3, 6, 12, 18, and 24 months after RFCA and whenever patients complained of palpitation following the 2012 HRS/EHRA/ECAS Expert Consensus Statement guidelines.10 We defined recurrence of AF as any episode of AF or atrial tachycardia of at least 30 sec in duration.17 If any ECG-documented AF episode occurred within the three-month blanking period during follow-up, patients were then diagnosed as an early recurrence, and any AF recurrence thereafter was diagnosed as clinical recurrence.17 In case of early recurrence and clinical recurrence, AADs were prescribed.
A total of 608 patients without evidence of AF recurrence on ECG and Holter monitoring at 3 months after RFCA were included in this study. We compared the thromboembolic events and major bleeding complications in 296 patients for whom warfarin was changed to 100 mg of aspirin (ASA group) and 312 patients for whom anticoagulation was maintained (W group) after confirming no recurrence in the 3rd-month post-RFCA Holter monitoring (Fig. 1). The primary endpoints were thromboembolism including ischemic stroke/transient ischemic attack (TIA) and major bleeding after 3 months from RFCA. Stroke was defined as symptomatic ischemic cerebral infarction with apparent brain lesion in imaging studies. TIA was defined as a transient episode of neurologic dysfunction confirmed by a neurologist without brain lesion in imaging studies, with spontaneous symptomatic recovery within 24 hours. Major bleeding events were defined as any type of hemorrhage requiring blood transfusion or intervention, and bleeding with reduction of hemoglobin levels by ≥4.0 g/dL.18 We compared incidences, hazard ratio and event-free survival of thromboembolic events (including stroke, TIA, and other thromboembolism) and major bleeding events between ASA and W groups depending on CHA2DS2-VASc score.
The results are expressed as mean±standard deviation. The Student's t-test, χ2 test were used for comparison of incidences of primary endpoints between the groups. Univariate and multivariate Cox regression analyses were used for comparison of hazard ratio (HR) between the groups. The assumption was assessed by log-minus-log-survival function and found that the proportion hazards assumption was reasonable. Parameters of a p value ≤0.1 by univariate analysis were included for multivariate analysis. HRs and 95% confidence intervals (CIs) were calculated. The Kaplan-Meier method was used for analyzing event-free survival. A p value <0.05 was considered significant. The data were analyzed using the Statistical Package for the Social Sciences version 20.0 (IBM Inc., Armonk, NY, USA).
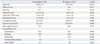
Patient demographic and clinical characteristics in the ASA and W groups at baseline were shown (Table 1). There were no significant differences in clinical profiles, LA size, and left ventricular function. CHADS2, CHA2DS2-VASc, and HAS-BLED scores were not significantly different between two groups. However, past history of stroke/TIA was more frequent in the W group (14.5%) than in the ASA group (7.1%, p=0.006). The time in the therapeutic range of international normalized ratio (INR) for the W group was 44.2% during the follow-up period. In sixty-seven patients (6.1%), AF recurred after 3 months of post-RFCA. After recurrence of AF, warfarin was restarted according to CHA2DS2-VASc score.
During the 18.0±12.2 months of follow-up, stroke occurred in a patient in the ASA group (0.3%). Stroke and TIA occurred in one and two patients in the W group, respectively (1.0%, p=0.342). All patients who experienced a stroke recovered without sequelae. In all patients with thromboembolic complications, heart rhythm was sinus rhythm at the time of stroke or TIA. Major bleeding events occurred in two patients in ASA and W groups (0.7% and 0.6%, respectively; p=0.958). The detailed information about events in each patient was shown (Table 2). There were no thromboembolic or major bleeding complications in patients with AF recurrence.
In univariate and multivariate Cox regression analyses, CHA2DS2-VASc score (HR, 3.80; 95% CI, 1.13-12.71; p=0.031) was an independent risk factor for thromboembolic events, and HAS-BLED score (HR, 3.90; 95% CI, 1.13-13.45; p=0.031) was an independent risk factor for major bleeding events (Table 3). By Kaplan-Meier analysis, there were no significant differences of event-free survival from the composite primary endpoint (thromboembolic and major bleeding events) between the groups (p=0.968) (Fig. 2A).
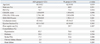
Among the 608 included patients, 259 patients (42.6%) had a CHA2DS2-VASc score ≥2 (ASA group, 121; W group, 138). Table 4 shows comparison of clinical characteristics of the patients with CHA2DS2-VASc score ≥2 in the ASA and W groups. The patients in the W group were younger (62.9±9.0 years vs. 65.5±8.3 years, p=0.019) and more likely to have had a history of stroke/TIA (32.6% vs. 17.3%, p=0.008) than the patients in the ASA group. In patients with CHA2DS2-VASc score ≥2, thromboembolic events occurred in one patient in the ASA group (0.8%) and three patients in the W group (2.2%, p=0.380), respectively during follow-up period. Major bleeding events occurred in one and two patients in the ASA (0.8%) and W groups (1.4%, p=0.640), respectively. By Kaplan-Meier analysis, there were no significant differences of event-free survival from the composite primary endpoint in the patients with CHA2DS2-VASc score ≥2 between the groups (p=0.822) (Fig. 2B).
This is a retrospective observational study that evaluated the risk and benefit of switching anticoagulation to aspirin after successful RFCA of AF, based on CHADS2, CHA2DS2-VASc, HAS-BLED scores, and recommended a standard rhythm monitoring follow-up strategy. The present study suggested that switching warfarin to aspirin 3 months after RFCA could be as safe and efficacious as continuous anticoagulation even in patients with a CHA2DS2-VASc score ≥2. However, strict and continuous rhythm monitoring and maintenance of optimal INR should be emphasized to achieve these results. We also proved that CHA2DS2-VASc and HAS-BLED scores were very valuable parameters in predicting ischemic stroke and hemorrhagic complications, even after RFCA of AF.
Although warfarin can significantly reduce stroke risk by 64% in patients with non-valvular AF,19 it still has an annual 1.6-2.4% risk of stroke or systemic embolism and an annual 3.1-3.4% risk of major hemorrhagic complications.11,12,13 Moreover, the annual risk of fatal hemorrhagic stroke is between 0.7-0.8%.11,12,13 Therefore, the 2010 ESC guidelines recommended caution, regular review, and correction of reversible bleeding risk factors for patients with a HAS-BLED score ≥3.5 In this study, 2 out of 721 patients experienced intracranial and spinal cord hemorrhages during anticoagulation, but they were not included in this analysis because these complications occurred within 3 months after RFCA. Although aspirin has a lower potential risk of major bleeding than warfarin, it reduces ischemic stroke risk by no more than 22% as compared with the 64% risk reduction achieved by anticoagulation.19 Therefore, aspirin can be considered in patients with a very low AF burden and a high HAS-BLED score. However, a previous study reported that the risk of intracranial hemorrhage was similar in patients taking aspirin to those taking warfarin, and that aspirin increases bleeding risk significantly when administered simultaneously with warfarin.20 In a large cohort study from Denmark including over 132000 patients with AF, warfarin was superior for stroke prevention and similar for bleeding risk compared to aspirin.21 Aspirin has rather higher bleeding risk than warfarin in Japan Atrial Fibrillation Stroke Trial (JAST).22 Discrepancies of current study from these two studies were that we included the patients with significantly reduced AF burden by catheter ablation, and those with young and low CHADS2 score. Generally, the risk of bleeding (HAS-BLED score) increases parallel with the risk of stroke (CHADS2 score), such as hypertension, old age, or previous stroke. In spite of ethnical similarity, major bleeding rates were 1.6% in JAST and 0.7% in current study. 2010 EHRA-EAPCI Consensus Documents recommended using warfarin monotherapy in patients with AF and percutaneous coronary intervention who are stable longer than 1 year.23 However, whether warfarin monotherapy is safe enough to prevent an annual 2.8% risk of very late stent thrombosis in patients with drug-eluting stents24 and major hemorrhagic complications remains unclear.
The main reason for continuing oral anticoagulation after successful RFCA is the concern for asymptomatic AF recurrence.10 Previous studies have shown a 0.5 to 7% risk of stroke or thromboembolic events after RFCA of AF,25,26,27 and current guidelines suggest anticoagulation should be maintained even after successful RFCA of AF according to CHADS2 or CHA2DS2-VASc score.10 However, there have been efforts to stop anticoagulation after potentially successful RFCA of AF because of the bleeding risks and inconvenience of warfarin. There have been several studies demonstrating incidence of thromboembolic events decrease after successful RFCA of AF.28,29,30 Oral, et al.25 showed that, among 755 successfully ablated AF patients, discontinuation of anticoagulation therapy was safe in patients younger than 65 years without a history of stroke. Bunch, et al.31 demonstrated the safety of 325 mg aspirin without warfarin in 690 patients with CHADS2 score 0 to 1, and Saad, et al.32 reported that no thromboembolic morbidity without anticoagulation existed in 327 patients with CHADS2 score ≤3. However, in a prior nonrandomized study of 3355 patients who underwent CPVI, suspension of oral anticoagulation therapy after successful RFCA was beneficial in patients at moderate to high risk of thromboembolism.30 Therefore the decision to anticoagulate after successful RFCA should be based on assessment of the risk-benefit ratio, and switching anticoagulation to aspirin can be considered in patients with high risk of hemorrhage and no recurrence of AF. There were two reasons that we grouped the patients based on anticoagulation therapy 3 months after RFCA. First, the current guidelines recommend that warfarin should be continued for 2 months after RFCA. Second, post-procedural 3 months are considered as blanking period and not considered as clinical recurrence.33
However, strict rhythm monitoring cannot be overemphasized. In this study, heart rhythm was sinus rhythm at the time of stroke or TIA in patients with thromboembolic complications. We might miss asymptomatic AF recurrence or non-cardioembolic source of stroke in these patients. Careful monitoring for rhythm is important in patients with high CHA2DS2-VASc or high HAS-BLED scores and patients at high risk of AF recurrence, such as those with high LA volume, PeAF, or long ablation time.34 In this study, we suggest that a regular rhythm monitoring strategy based on the current guidelines was acceptable for making a clinical decision of switching warfarin to aspirin. We also implied that CHA2DS2-VASc and HAS-BLED scores were still independent predictors for stroke/TIA and major hemorrhage after successful catheter ablation of AF.
This study was designed as a retrospective observational study, but not as a randomized study. Although CHADS2 and CHA2DS2-VASc scores were not significantly different between two groups, past history of stroke or TIA were higher in W group than in ASA group. It may be because the physicians tended to continue warfarin in patients with a history of stroke or TIA. Although we tried to follow the consensus guidelines for AF rhythm monitoring strictly, all silent recurrence of AF could not be detected on surface ECG or Holter monitoring. The time in therapeutic range was relatively low (44.2%) in the W group compared to previous large scale clinical trials, and it could be one of factors for no significant differences in thromboembolic complication between W group and ASA group. Although recovery of LA contractile function is an important factor for prediction of thromboembolic events, we did not analyze LA contractile function in the current study.
In conclusion, after successful RFCA of AF, switching warfarin to aspirin 3 months after RFCA could be as safe and efficacious as long-term anticoagulation, even in patients with CHA2DS2-VASc score ≥2. Strict heart rhythm monitoring in the aspirin group and careful INR monitoring in the anticoagulation group are important in patients after RFCA of AF. Future large randomized trials are warranted.
Figures and Tables
Fig. 1
Flow diagram and numbers of patients. AF, atrial fibrillation; RFCA, radiofrequency catheter ablation.

Fig. 2
Thromboembolic and major bleeding event-free survival by Kaplan-Meier method in all patients (A) and the patients with CHA2DS2-VASc score ≥2 (B) in ASA (solid line) and W (dotted line) groups.

ACKNOWLEDGEMENTS
This work was supported by a grant (A085136) from the Korea Health 21 R&D Project, Ministry of Health and Welfare, and a grant (2010-0010537) from the Basic Science Research Program of the National Research Foundation of Korea under the Ministry of Education, Science and Technology of the Republic of Korea.
References
1. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002; 347:1825–1833.

2. Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002; 347:1834–1840.

3. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001; 285:2864–2870.

4. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010; 137:263–272.

5. European Heart Rhythm Association. European Association for Cardio-Thoracic Surgery. Camm AJ, Kirchhof P, Lip GY, Schotten U, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010; 31:2369–2429.
6. Lee BH, Park JS, Park JH, Park JS, Kwak JJ, Hwang ES, et al. The effect and safety of the antithrombotic therapies in patients with atrial fibrillation and CHADS score 1. J Cardiovasc Electrophysiol. 2010; 21:501–507.

7. Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005; 293:2634–2640.

8. Jaïs P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008; 118:2498–2505.
9. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012; 367:1587–1595.

10. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012; 9:632–696.
11. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151.

12. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883–891.

13. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981–992.

14. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010; 138:1093–1100.

15. Verma A, Mantovan R, Macle L, De Martino G, Chen J, Morillo CA, et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. Eur Heart J. 2010; 31:1344–1356.

16. Pak HN, Oh YS, Lim HE, Kim YH, Hwang C. Comparison of voltage map-guided left atrial anterior wall ablation versus left lateral mitral isthmus ablation in patients with persistent atrial fibrillation. Heart Rhythm. 2011; 8:199–206.

17. European Heart Rhythm Association (EHRA). European Cardiac Arrhythmia Scoiety (ECAS). American College of Cardiology (ACC). American Heart Association (AHA). Society of Thoracic Surgeons (STS). Calkins H, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007; 4:816–861.

18. Kastrati A, Neumann FJ, Mehilli J, Byrne RA, Iijima R, Büttner HJ, et al. Bivalirudin versus unfractionated heparin during percutaneous coronary intervention. N Engl J Med. 2008; 359:688–696.

19. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146:857–867.

20. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012; 33:1500–1510.

21. Olesen JB, Lip GY, Lindhardsen J, Lane DA, Ahlehoff O, Hansen ML, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: A net clinical benefit analysis using a 'real world' nationwide cohort study. Thromb Haemost. 2011; 106:739–749.

22. Sato H, Ishikawa K, Kitabatake A, Ogawa S, Maruyama Y, Yokota Y, et al. Low-dose aspirin for prevention of stroke in low-risk patients with atrial fibrillation: Japan Atrial Fibrillation Stroke Trial. Stroke. 2006; 37:447–451.

23. Lip GY, Huber K, Andreotti F, Arnesen H, Airaksinen JK, Cuisset T, et al. Antithrombotic management of atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing coronary stenting: executive summary-a Consensus Document of the European Society of Cardiology Working Group on Thrombosis, endorsed by the European Heart Rhythm Association (EHRA) and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2010; 31:1311–1318.

24. Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007; 356:989–997.

25. Oral H, Chugh A, Ozaydin M, Good E, Fortino J, Sankaran S, et al. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation. 2006; 114:759–765.

26. Nademanee K, Schwab MC, Kosar EM, Karwecki M, Moran MD, Visessook N, et al. Clinical outcomes of catheter substrate ablation for high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2008; 51:843–849.

27. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005; 111:1100–1105.

28. Yagishita A, Takahashi Y, Takahashi A, Fujii A, Kusa S, Fujino T, et al. Incidence of late thromboembolic events after catheter ablation of atrial fibrillation. Circ J. 2011; 75:2343–2349.

29. Corrado A, Patel D, Riedlbauchova L, Fahmy TS, Themistoclakis S, Bonso A, et al. Efficacy, safety, and outcome of atrial fibrillation ablation in septuagenarians. J Cardiovasc Electrophysiol. 2008; 19:807–811.

30. Themistoclakis S, Corrado A, Marchlinski FE, Jais P, Zado E, Rossillo A, et al. The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol. 2010; 55:735–743.

31. Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, et al. Warfarin is not needed in low-risk patients following atrial fibrillation ablation procedures. J Cardiovasc Electrophysiol. 2009; 20:988–993.

32. Saad EB, d'Avila A, Costa IP, Aryana A, Slater C, Costa RE, et al. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score ≤3: a long-term outcome study. Circ Arrhythm Electrophysiol. 2011; 4:615–621.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download