Abstract
Purpose
The aim of this study was to determine the force distribution and pattern of mastication after injection of botulinum toxin type A (BTX-A) into both masseter muscles. The hypothesis to be tested was that the difference between right and left balance of occlusal force diminishes over time following BTX-A injection.
Materials and Methods
Fifteen patients were submitted to BTX-A injection therapy for subjective masseter hypertrophy. A total of 25 U of BTX-A (50 U in total) was injected into two points located 1 cm apart at the center of the lower one-third of both masseter muscles. All patients were examined using the T-Scan occlusion analysis system before and 4, 8, 12, and 24 weeks after BTX-A injection.
Results
A significant change in force balance was found between the right and left sides over time and the difference between the two sides decreased with the time post-injection, reaching a minimum at 12 weeks. Comparison of the force balance between the anterior and posterior occlusions revealed no significant difference at any of the time points. The occlusion and disclusion times (right and left sides) did not differ significantly with time since BTX-A injection.
The clinical use of botulinum toxin type A (BTX-A) has expanded into the field of dentistry over the past decade. It is used in the treatment of masticatory and facial muscle spasm, severe bruxism, facial tics, orofacial dyskinesias, dystonias, and idiopathic hypertrophy of the masticatory muscles,1 as well as in the treatment of temporomandibular disorders,2 myofascial pain syndrome,3 headaches such as chronic migraine,4 recurrent dislocation of the temporomandibular joint (TMJ), drooling, and Frey's syndrome.5 Application of BTX-A to various orofacial regions in patients often requires injection into masticatory muscles such as the masseter and temporalis muscles, which can cause temporary muscle paralysis, weakness, and atrophy.
The control of mastication is dependent in large part upon sensory feedback, which involves epithelial mechanoreceptors, periodontal, TMJ, and muscle afferents.6 Changes in the afferent input from a muscle caused by injection of BTX-A can modify the response of the cortex and the motor neuron activity, and even initiate the activity of irrelevant muscles. Such injections into masticatory muscles can subsequently influence mastication directly by inducing muscle weakness and atrophy, as well as indirectly by influencing the central pattern generator in the brainstem via modification of the sensory feedback from the masticatory muscle spindle.7
It has been often observed that BTX-A has analgesic effects and reduces hyperactivity in the injected muscle.8 However, in our clinic, some patients with pretreatment unilateral chewing habits report an equalization of masticatory force after BTX-A injection. This effect of BTX-A has yet to be studied in detail.
The T-Scan occlusion analysis system is a dental tool that is used to analyze masticatory force. It was first devised in 1984 to measure occlusal forces and contact times as a prosthodontic adjunct in the treatment of occlusal problems and temporomandibular disorders. It has also been used as a measurement guide during prosthetic insertion and occlusal adjustment procedures. Data on occlusal forces and contact times are gathered by a recording sensor in the T-Scan system,9 and can subsequently be visualized in movie format, providing definitive diagnostic imaging of the force balance and function of the masticatory muscles.10
The aim of this study was to determine the force distribution and pattern of mastication after injection of BTX-A into the bilateral masseter muscles. The hypothesis to be tested was that the difference between right and left balance of occlusal force diminishes over time following BTX-A injection.
This study was approved by the Institutional Review Board committee of Yonsei School of Dentistry (No. 2-2011-0024). Twenty-four patients volunteered for this study and fifteen patients were selected during the screening process. These patients had BTX-A injection therapy for subjective masseter hypertrophy, and T-Scan examination for this study. Only subjects with normal occlusion conditions (class I) were included. Those with abnormal occlusion conditions that could affect the normal occlusion (e.g., missing teeth or severe tooth attrition) were excluded. Also those who had undergone dental treatment, temporomandibular disorder including TMJ osteoarthritis, and occlusal interference during eccentric movement were excluded because it could also disturb the normal occlusion. Additionally, those who were pregnant or had injection of BTX-A during the previous 6 months were excluded because those condition could affect the results of the study.
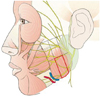
The BTX-A used in this study was Botox (Allergan, Irvine, CA, USA); 100 U of Botox, obtained as a freeze-dried powder, was reconstituted to a concentration of 5 U/0.1 mL using 2 mL of 0.9% sterile, nonpreserved saline, and used immediately after reconstitution. A total of 25 units of BTX-A was injected into both masseter muscle bilaterally using a 1-mL syringe with a 29-gauge, 1/2-inch-long needle. Areas of masseter prominence on clenching were marked, and injected at two points at the center of the lower one-third of the masseter muscle separated by 1 cm (Fig. 1). This site was chosen to avoid accidental toxin injection into the parotid gland, parotid duct, or facial artery.11 The injection was conducted by a single person in order to reduce error range.
All patients were examined using the T-Scan occlusion analysis system (T-Scan III, Yours Dental, Seoul, Korea) before and 4, 8, 12, and 24 weeks after BTX-A injection. This T-Scan system comprises Microsoft-Windows-based software, the associated hardware, and patented paper-thin disposable sensors.10 The information provided on T-Scan movies is listed in Table 1.
The sensor associated with the T-Scan system consists of two layers of Mylar (reinforced polyester film) in the form of a laminated pressure-sensitive ink grid. The film is covered by a silver-thread grid, the intersection points of which are bathed in conductive ink. When a patient closes firmly on the sensor, the resultant reduction in electric resistance is converted to 8-bit digital values and translated into an image on the screen.12
The patient was seated in an upright position with because the supine position can alter the contact position.13 The T-Scan III sensor and sensor support assembly were inserted intraorally and positioned correctly. The T-Scan force-movie mode was then activated manually by pushing the button on the handle. The patients were instructed to bite in the habitual intercuspal position, and then to make excursive movements (not guided by the investigator). The right and left excursions were recorded separately. The patients were allowed a few minutes of rest between recordings. All of these procedures were repeated three times; the first two recordings were performed to familiarize the patient with the procedure, and the third record, made when the patient was completely familiar with the protocol, was used for the data analysis.
This study measured the distribution of forces between the left and right sides and between the anterior and posterior regions during clenching, and the change in occlusion and disclusion times after BTX-A injection (Figs. 2 and 3). The disclusion time, in seconds, is required to disclude the working and nonworking molar interferences and nonworking premolar interferences from the habitual centric closure position to the completion of a mandibular excursion, and the occlusion time is the time in seconds, from the first contact of the teeth to the maximal intercuspation.14
Linear mixed modeling for longitudinal data was conducted to analyze the change in the measured parameters over time. The interaction between times and groups was evaluated by compound symmetry covariance structure, banded Toeplitz, or autoregressive covariance structure. Bonferroni's correction was used for post-hoc analysis. A p value of <0.01 was considered indicative of statistical significance. All statistical analyses were performed using the SAS Windows statistics program (version 9.2, SAS Institute, Cary, NC, USA).
A significant change in force balance was found between the right and left sides over time (i.e., preinjection and 4, 8, 12, and 24 weeks post-BTX-A injection) and at each measurement time point (p<0.0001). The difference between the two sides decreased with the time postinjection, reaching a minimum at 12 weeks (Fig. 4). These findings reflect the well-established time course of the actions of BTX-A.15 In other words, according to this result, the left and right clenching force becomes more balanced with the increasing effect of botulinum toxin injection.
Comparison of the force balance between the anterior and posterior occlusions revealed no significant difference at any of the time points (i.e., preinjection and 4, 8, 12, and 24 weeks postinjection; p>0.01 for all). Furthermore, comparison of the preinjection force balance with those measured at 4, 8, 12, and 24 weeks postinjection revealed no significant difference at any time point (p>0.05 for all) (Table 2). In other words, there is no correlation between the balance of anterior and posterior masticatory force and the increasing effect of botulinum toxin injection.
The occlusion and disclusion times (right and left sides) did not differ significantly with time after BTX-A injection (p>0.01) (Table 3).
Benign masseter hypertrophy is a relatively uncommon condition that can occur unilaterally or bilaterally. Unilateral or bilateral hypertrophy of the masseter muscle is characterized by an increase in the volume of the muscle mass. This condition is benign, asymptomatic, and must be differentiated from parotid gland disease odontogenic problems, and rare neoplasms of muscular tissue. The reasons why patients request a medical consultation are predominantly related to aesthetics, especially if the hypertrophy is unilateral due to a noticeable asymmetry of the lower third of the face.16,17 This study was intended for patients who complained only about masseter hypertrophy, and not other symptoms.
Botulinum toxin is a potent biological toxin produced by the Gram-positive bacterium Clostridium botulinum.18 It is a presynaptic neurotoxin that causes dose-dependent weakness or paralysis in skeletal muscles by blocking the calcium-mediated release of acetylcholine from motor nerve endings.19,20 BTX-A, one of seven subtypes of the toxin, is a dichain protein consisting of a 50-kD light chain linked to a 100-kD heavy chain by a disulfide bond; it functionally denervates the affected portions of the muscle and it primarily affects α-motor neuron function, but it may also affect the γ motor neurons in the muscle spindles.21 Local paralysis is reversed chiefly by neural sprouting, effectively reinnervating the muscle.22 Long-term reductions in α, γ, and Ia neuronal activity may have indirect effects on the central nervous system. This was demonstrated when Moreno-López, et al.23 who showed that single injections of BTX-A into the lateral rectus muscle of cats caused inhibition of abduction, altered electromyographic signals in the contralateral ocular muscles, and a disruption of the abducens motor neuron discharge patterns that lasted longer than 2 months.
BTX-A may also act directly or indirectly on nociceptors that affect transmission of sensory signals through A-δ and C fibers, and impact the detection of sensory signals through mechanoreceptors and chemo-nociceptors. These findings may also explain the changes in the distribution of clenching forces after BTX-A injection reported herein. It is possible that the redistribution of clenching force after BTX-A injection is due to changes both in the chewing pattern at the central nervous system level and atrophy and weakness of the peripheral muscle region. However, more detailed research into the exact mechanism underlying the change in chewing pattern at the level of the central nervous system is needed to clarify this issue.
As mentioned above, as the medicinal effects of BTX-A increase, there is a concomitant decline in the difference in clenching force between the left and right sides post injection. BTX-A injection shows maximum effect at 12 weeks postinjection, and after, that the effect decreases possibly creating an imbalance in force balance once again, which is suspected to be caused by the patients chewing habit.
Furthermore, there was no significant change in either occlusion or disclusion time after BTX-A injection. The theory that decline in the difference in clenching force caused by the effect of botulinum toxin on sensory transmission by A-δ and C fibers, either directly or indirectly, is supported by the results of the present study. The durations of occlusion and disclusion times are determined by interference between the teeth. However, since botulinum toxin cannot influence that interference, it is perhaps not surprising that there was no significant change in either duration following BTX-A injection. Furthermore, the much lighter contact between the upper and lower anterior teeth than between the upper and lower posterior teeth in normal occlusion, and the absence of an effect of botulinum toxin on the contact between the anterior teeth explain why no significant difference was found between the anterior and posterior balance over time post-BTX-A injection. It may be that if all of the subjects had possessed a full-contact arch, a difference may have been observed between the balance of anterior and posterior occlusions after BTX-A injection.
This study was subject to several limitations. First, some of the patients were already acclimatized to the T-Scan procedure after the first recording, and so their clenching velocity or lateral excursions may have increased with repeated recordings compared to the other, non-acclimatized patients. While this phenomenon is likely to have had little effect on the distribution of clenching force, it may have had a slight effect on occlusion and disclusion times. The data from the third recording, rather than the mean of the three measured values, were therefore analyzed since it was felt that the data obtained at the first and second recordings would be inaccurate. Second, since there was no control group, it was not possible to determine whether there was a placebo effect. Future studies should address these limitations.
In conclusion, fifteen patients were examined using the T-Scan system before and after BTX-A injection into the masseter muscles. A decline in the difference in the clenching force between the left and right sides was found with increasing time (and hence increasing medicinal effect) up to 12 weeks following BTX-A injection.
Figures and Tables
Fig. 2
Balance of clenching force in a T-Scan movie (A: divided into two parts: left and right sides, B: divided into four parts labeled in clockwise direction: anterior left, anterior right, posterior right, posterior left).

Fig. 3
(A) Occlusion time. (B) Disclusion time. (A) A1 is the point the teeth start to occlude, B1 is the point the dentition is fully occluded, and the difference of A1 and B1 is occlusion time, marked as OT. (B) C1 is the point lateral movement starts, D1 is the point the dentition is fully separated and the difference is disclusion time, marked as DT. OT, occlusion time; DT, disclusion time.

Fig. 4
Force distribution on right and left sides before BTX-A injection (A) and 12 weeks thereafter (B). (A) In the left figure, before botulinum toxin injection the distribution of the left and right force was uneven, 72.1% and 27.9%. 12 weeks post injection, (B) the right figure, the distribution evened as left 58.1% and right 27.9%. BTX-A, botulinum toxin type A.

References
1. Clark GT. The management of oromandibular motor disorders and facial spasms with injections of botulinum toxin. Phys Med Rehabil Clin N Am. 2003; 14:727–748.

2. Freund B, Schwartz M, Symington JM. Botulinum toxin: new treatment for temporomandibular disorders. Br J Oral Maxillofac Surg. 2000; 38:466–471.

3. Lang AM. Botulinum toxin therapy for myofascial pain disorders. Curr Pain Headache Rep. 2002; 6:355–360.

4. Lovell BV, Marmura MJ. New therapeutic developments in chronic migraine. Curr Opin Neurol. 2010; 23:254–258.

5. Bhogal PS, Hutton A, Monaghan A. A review of the current uses of Botox for dentally-related procedures. Dent Update. 2006; 33:165–168.

6. Soboļeva U, Lauriņa L, Slaidiņa A. The masticatory system--an overview. Stomatologija. 2005; 7:77–80.
7. Okeson JP. Management of temporomandibular disorders and occlusion. 6th ed. St. Louis, Missouri: Elsevier Mosby Publishers;1993.
8. Fallah HM, Currimbhoy S. Use of botulinum toxin A for treatment of myofascial pain and dysfunction. J Oral Maxillofac Surg. 2012; 70:1243–1245.

9. Kerstein RB, Lowe M, Harty M, Radke J. A force reproduction analysis of two recording sensors of a computerized occlusal analysis system. Cranio. 2006; 24:15–24.

10. Montgomery MW, Shuman L, Morgan A. T-scan dental force analysis for routine dental examination. Dent Today. 2011; 30:112–114. 116
11. Hu KS, Kim ST, Hur MS, Park JH, Song WC, Koh KS, et al. Topography of the masseter muscle in relation to treatment with botulinum toxin type A. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 110:167–171.

12. Kerstein RB. Combining technologies: a computerized occlusal analysis system synchronized with a computerized electromyography system. Cranio. 2004; 22:96–109.

13. Makofsky HW, Sexton TR, Diamond DZ, Sexton MT. The effect of head posture on muscle contact position using the T-Scan system of occlusal analysis. Cranio. 1991; 9:316–321.

14. Kerstein RB, Wright NR. Electromyographic and computer analyses of patients suffering from chronic myofascial pain-dysfunction syndrome: before and after treatment with immediate complete anterior guidance development. J Prosthet Dent. 1991; 66:677–686.

15. Aoki KR, Ranoux D, Wissel J. Using translational medicine to understand clinical differences between botulinum toxin formulations. Eur J Neurol. 2006; 13:Suppl 4. 10–19.

16. Addante RR. Masseter muscle hypertrophy: report of case and literature review. J Oral Maxillofac Surg. 1994; 52:1199–1202.

17. Rispoli DZ, Camargo PM, Pires JL Jr, Fonseca VR, Mandelli KK, Pereira MA. Benign masseter muscle hypertrophy. Braz J Otorhinolaryngol. 2008; 74:790–793.

18. Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981; 33:155–188.
19. Drachman DB. Atrophy of skeletal muscle in chick embryos treated with botulinum toxin. Science. 1964; 145:719–721.

20. Melling J, Hambleton P, Shone CC. Clostridium botulinum toxins: nature and preparation for clinical use. Eye (Lond). 1988; 2(Pt 1):16–23.

21. Filippi GM, Errico P, Santarelli R, Bagolini B, Manni E. Botulinum A toxin effects on rat jaw muscle spindles. Acta Otolaryngol. 1993; 113:400–404.

22. Holds JB, Alderson K, Fogg SG, Anderson RL. Motor nerve sprouting in human orbicularis muscle after botulinum A injection. Invest Ophthalmol Vis Sci. 1990; 31:964–967.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download