Abstract
Purpose
Epidural analgesia has been the preferred analgesic technique after major abdominal surgery. On the other hand, the combined use of intrathecal morphine (ITM) and intravenous patient controlled analgesia (IVPCA) has been shown to be a viable alternative approach for analgesia. We hypothesized that ITM combined with IVPCA is as effective as patient controlled thoracic epidural analgesia (PCTEA) with respect to postoperative pain control after conventional open gastrectomy.
Materials and Methods
Sixty-four patients undergoing conventional open gastrectomy due to gastric cancer were randomly allocated into the intrathecal morphine combined with intravenous patient-controlled analgesia (IT) group or patient-controlled thoracic epidural analgesia (EP) group. The IT group received preoperative 0.3 mg of ITM, followed by postoperative IVPCA. The EP group preoperatively underwent epidural catheterization, followed by postoperative PCTEA. Visual analog scale (VAS) scores were assessed until 48 hrs after surgery. Adverse effects related to analgesia, profiles associated with recovery from surgery, and postoperative complications within 30 days after surgery were also evaluated.
Results
This study failed to demonstrate the non-inferiority of ITM-IVPCA (n=29) to PCTEA (n=30) with respect to VAS 24 hrs after surgery. Furthermore, the IT group consumed more fentanyl than the EP group did (1247.2±263.7 µg vs. 1048.9±71.7 µg, p<0.001). The IT group took a longer time to ambulate than the EP group (p=0.021) and had higher incidences of postoperative ileus (p=0.012) and pulmonary complications (p=0.05) compared with the EP group.
Recently, the combined use of intrathecal morphine (ITM) and intravenous patient controlled analgesia (IVPCA) has been shown to be a viable alternative approach for analgesia after major abdominal surgery.1,2 Several studies reported that ITM-IVPCA offered similar or even better analgesia compared to patient controlled epidural analgesia in patients undergoing hepato-pancreato-biliary surgery3 or laparoscopic colorectal surgery.4 Furthermore, there were reports that ITM-IVPCA compared to epidural analgesia is associated with a lower or similar incidence of respiratory complication and a better recovery profile such as shorter hospital stay in hepato-pancreato-biliary surgery3 or laparoscopic colorectal surgery.4,5
On the other hand, epidural analgesia remained the preferred analgesic technique in patients undergoing major abdominal surgery because it provides good pain control,6 improved respiratory function,7 and decreased chest-related morbidity.8 For epidural analgesia in patients undergoing conventional open gastrectomy, an epidural catheter is recommended to be placed at the level of the thoracic spine. Inserting a epidural catheter at the thoracic level is known to be procedurally difficult.9 Additionally, neurological complications related to neuraxial blockade were reported to occur more often after an epidural than spinal block.10 Therefore, if, in patients undergoing gastrectomy, the ITM-IVPCA could provide similar analgesia and recovery profile, compared to patient-controlled thoracic epidural analgesia (PCTEA), it could be the preferred analgesic option in conventional open gastrectomy. However, to the best of our knowledge, the efficacy of ITM-IVPCA in patients undergoing gastrectomy due to gastric cancer has not yet been investigated.
We hypothesized that ITM combined with IVPCA is as effective as PCTEA with respect to postoperative pain control after conventional open gastrectomy for stomach cancer. To test this hypothesis, we performed the present study designed as a randomized, non-inferiority clinical trial, since the aim was not to demonstrate superiority of one analgesic regime, but to prove that ITM-IVPCA is not worse than PCTEA.
After obtaining the approval of the Institutional Review Board of our institution, participants were recruited from the Anesthesiology Preoperative Evaluation Clinic and provided written informed consent. This investigation was conducted at a single tertiary medical center in Seoul, Korea between September 2010 and August 2011. Sixty-four patients (aged ≥20 yrs, American society of anesthesiologists physical status I-II) scheduled to undergo conventional open gastrectomy for stomach cancer were included. The exclusion criteria were as follows: presence of spinal deformity or spinal disease; pulmonary, hepatic or renal disease; any contraindication to epidural or intrathecal injection; allergy to fentanyl and local anesthetics; inability to understand the pain scale; and any type of chronic pain or current opioid use. After written informed consent was obtained, patients were instructed on how to use the device for patient-controlled analgesia (PCA) and how to report pain via the visual analogue scale (VAS) the day before surgery.
By using a table of random sampling numbers, patients were randomly allocated into either the intrathecal morphine combined with intravenous patient-controlled analgesia (IT) group (receiving ITM-IVPCA, n=32) or patient-controlled thoracic epidural analgesia (EP) group (receiving PCTEA, n=32) on the day of the surgery.
In the EP group, an epidural catheter was placed before the induction of general anesthesia. Under standard monitoring, the epidural catheter was inserted at the level of T8-T9 or T9-T10 via a 17-gauge Touhy needle and advanced 5 cm into the epidural space. Intravascular or subarachnoid placement of the epidural catheter was ruled out by confirming that no blood or cerebrospinal fluid was aspirated. Also, 3 mL of 1% lidocaine was administered. If there was no rapid onset of neuroaxial block, suggesting intrathecal delivery of the local anesthetic, the placement of the epidural catheter was completed. After 30 min, sensory block around the level of T8-T10 was tested by pinprick. Before the induction of anesthesia, 5 mL of a mixture of 0.2% ropivacaine and 4 µg/mL of fentanyl was administered through the epidural catheter. It was administered once more upon peritoneal closure, and then a pump for patient-controlled analgesia (Accufusor Plus®, Woo Young Medical Co., Ltd., Seoul, Korea), containing a mixture of 0.2% ropivacaine and 4 µg/mL of fentanyl (total volume, 250 mL), was connected to the epidural catheter. The basal infusion of the pump was set at 5 mL/hr with a 0.5 mL bolus doses allowed every 15 min.
In the IT group, 0.3 mg of morphine in 0.9% saline solution (total volume 4 mL) was administered into the intrathecal space via a 27-gauge pencil-point spinal needle placed at the level of L3-L4, before the induction of general anesthesia. Upon peritoneal closure, 1 µg/kg of fentanyl was administered intravenously, and the same pump used in the EP group containing 20 µg/kg of fentanyl in 0.9% saline solution (total volume 250 mL) was connected. IVPCA was then initiated. The patients in the IT group thus received basal infusion of fentanyl at 0.4 µg/kg/hr with boluses of 0.04 µg/kg and a lockout period of 15 min. Because fentanyl has a relatively short half-life, and thus duration of analgesia of boluses is brief, we used the continuous infusion for the IVPCA. In both groups, the infusate for PCA were prepared by an anesthesiologist who did not participate in the postoperative assessment.
To evaluate patient discomfort related to the procedure, we assessed the duration of the procedure (from infiltration of local anesthetic to removal of the Tuohy needle or spinal needle) and subjective grade of discomfort that the patient suffered during the procedure (1, minimal; 2, mild; 3, moderate; 4, severe) for both groups.
Additional intravenous administration of fentanyl (0.4 µg/kg) upon patient demand was allowed for further analgesia in both groups. After administration of additional fentanyl, respiration rate and the level of consciousness were closely monitored for 30 min. In both groups, patients with persistent insufficient analgesia (VAS >40) at 30 min after post-anesthesia care unit admission were considered as a technical failure of epidural or spinal analgesia and thus excluded from the study. In these patients, a different technique for analgesia was applied.
Anesthesia was performed according to the same standard protocol in both groups. All patients were premedicated with 0.1 mg of glycopyrrolate IV. Anesthesia was induced with 1.5 mg/kg of propofol and 1 µg/kg of remifentanil. For tracheal intubation, 0.6 mg/kg of rocuronium was given. Thereafter, the patient was mechanically ventilated to maintain end-tidal carbon dioxide at 35±5 mm Hg during the surgery. Anesthesia was maintained with 0.6-1.2 minimum alveolar concentration end tidal desflurane in an air-oxygen mixture (fraction of inspired oxygen=0.5) and 0.05-0.2 µg/kg/min remifentanil infusion. The concentration of end-tidal desflurane and the infusion rate of remifentanil were adjusted according to clinical parameters (blood pressure or heart rate within 20% of the baseline). SpO2, heart rate, and noninvasive blood pressure were monitored and recorded throughout the surgery. For prevention of postoperative nausea and vomiting, 0.3 mg of ramosetron was administered intravenously at peritoneal closure.
An anesthesiologist blinded to group allocation evaluated the postoperative assessments throughout the study period. Blinding of the allocated group was ensured because the connected part of the PCA pump to the epidural catheter or the intravenous line was fixed to the patient and covered by the patient's clothing. Moreover, the PCA pump was in a protective bag shielding the medication label on the pump. The assessor visited the patients at 1, 3, 6, 24, and 48 hrs after surgery and assessed VAS of the maximum pain experienced at rest and upon coughing (or moving) since last questioning (or recovery from anesthesia). Besides the PCA, the total additional fentanyl administration was also evaluated.
Postoperative respiratory depression was defined as less than or equal to 8 breaths/min. Postoperative sedation was evaluated using the 8-point modified Ramsey Sedation Scale (RSS), and oversedation was defined as RSS greater than 4. Patients were excluded from the study and treated with naloxone if they had respiratory depression or oversedation persisting for more than 1 hour. Other adverse effects of analgesia such as nausea, vomiting, pruritus, and hypotensive episode were evaluated until 48 hr after surgery. An objective motor block was assessed using Bromage score (0=full movement, 1=inability to perform straight leg raise in either leg, 2=inability to flex either knee, 3=inability to flex either ankle) at 6, 24, and 48 hr after surgery. Time to ambulation, eating, and flatus was also assessed to compare the recovery profile after surgery.
Postoperative management was left to the discretion of the Yonsei Gastric Cancer Clinic team. The total amounts of intravenous fluid, except total parenteral nutrition, administered during the postoperative 48 hr were recorded. The incidence of postoperative complications within postoperative day (POD) 30 were assessed, which were also diagnosed and treated by the Yonsei Gastric Cancer Clinic team; intraperitoneal fluid collection, intraluminal bleeding, postoperative ileus, anastomosis site leakage, pancreatitis, pulmonary atelectasis or pleural effusion, pancreatitis and urinary complications were assessed following their protocols.11
Duration of hospital stay and mortality within 30 days after operation were recorded as postoperative outcome.
The primary outcome variable was a VAS score at rest and upon coughing 24 hrs after surgery. Therefore, the number of patients required for each group was determined according to VAS score at 24 hrs after surgery on the basis of the non-inferiority hypothesis. For non-inferiority of the IT group versus the EP group, a maximum difference of 10 (margin of non-inferiority) on the VAS scale was considered acceptable as statistically significant changes of 10 in VAS scores are clinically significant in a variety of psychophysiological measurements,12 whereas small differences between VAS scores may be statistically significant but clinically meaningless.13 On the basis of previously published data,14 a standard deviation of 13.6 was assumed for VAS distribution. Under these conditions, power analysis indicated that a sample size of 29 patients for each group would be required with α=0.025 (one-sided hypothesis) and a power of 80%. Taking into consideration the potential for drop-outs, we decided to enroll 32 patients per group.
The primary outcome variable was analyzed according to a non-inferiority approach. The 95% confidence intervals of the mean differences in VAS scores, at rest and on coughing, between the two treatments 24 hrs after surgery were calculated and shown in relation to the predefined margin of inferiority.
Where appropriate, results were shown as mean (SD) or median (interquartile range, IQR). Comparisons of VAS scores, total fentanyl consumption, and recovery profiles between the two groups were conducted using unpaired Student's t-tests. Differences between the two groups regarding the grade of discomfort during the procedure and duration of hospital stay were analyzed by the Mann-Whitney rank-sum test and the incidences of adverse effects, postoperative complications, and postoperative outcomes were compared using χ2 test or Fisher's exact test. For these variables, a p-value of <0.05 was considered to be statistically significant. PASW statistics 18 (SPSS Inc., Chicago, IL, USA) and SAS software version 9.2 (SAS, Cary, NC, USA) were used to conduct the aforementioned statistical analyses.
A total of 64 patients were randomly allocated to one of the two groups (Fig. 1). Among patients in the IT group, one was excluded because of persistent oversedation and two were excluded due to persisting insufficient analgesia. In the EP group, two patients were excluded because of failure with placing the epidural catheter and persistent insufficient analgesia. The two groups were comparable in terms of patient characteristics, calculated comorbidity index15 and data collected during the perioperative period (Table 1).
Among the procedure related profiles for intrathecal injection or epidural catheter placement, length of procedure was significantly shorter in the IT group than the EP group [4.1±2.4 (min) vs. 6.7±3.2 (min), mean±SD, p=0.013]. Grade of discomfort during the procedure was lower in the IT group than the EP group [2 (1-2) vs. 3 (2-3), median (IQR), p=0.004].
VAS scores at rest (Fig. 2A) and on coughing (Fig. 2B) until 48 hr after surgery were statistically comparable for both the IT and EP groups. However, the 95% confidence interval of the mean differences in VAS scores between the two groups at rest and on coughing at 24 hrs after surgery included the upper margin of equivalence, and thus, this study failed to demonstrate non-inferiority of intrathecal morphine administration to PCTEA (Fig. 3). The total fentanyl consumption up to 48 hrs postoperatively was significantly greater in the IT group than the EP group (1247.2±263.7 µg vs. 1048.9±71.7 µg, p<0.001).
The adverse effects of analgesia are presented in Table 2. Postoperative nausea developed more frequently in the IT group. No motor blockade was detectable in any patient during the 48 hr postoperative period (all Bromage score 0). Total amounts of administered fluid during the postoperative period of 48 hr were comparable between the two groups (4517.0±913.2 mL in the group EP vs. 4666.3±975.6 mL in the group IT, mean±SD). Regarding the recovery profiles from the surgery, the IT group took 12 hr longer to ambulate than the EP group (p=0.02). However, there were no differences in time to eating and flatus (Table 3).
We assessed the postoperative complications and outcomes documented within POD 30 (Table 4). In the IT group, postoperative ileus (p=0.012) and pulmonary complications (p=0.05) developed more frequently, compared to the EP group. However, although the incidence of postoperative complications was higher in the IT group than in the EP group, there were no statistically significant differences in hospital stay (p=0.14). Moreover, there was no mortality within POD 30 in both groups.
The present study revealed that the analgesic effect of single intrathecal morphine injection combined with IVPCA is not as effective as the analgesic effect of PCTEA in patients undergoing gastrectomy. The treatment differences in terms of VAS scores failed to demonstrate the non-inferiority of ITM-IVPCA at 24 hrs after the operation compared to PCTEA, despite no significant difference in VAS scores. However, the IT group consumed a larger amount of fentanyl than the EP group did. Furthermore, ITM-IVPCA was associated with a longer time to ambulation, and, eventually, an increased incidence of postoperative ileus and pulmonary complication.
In a meta-analysis, a wide range of dose of intrathecal morphine (0.1-4 mg) have been tested in various types of surgery.16 According to recent reports, intrathecal administration of 0.2-0.4 mg of morphine improves postoperative analgesia without respiratory depression after major abdominal surgery.1,17 Therefore, we selected 0.3 mg for intrathecal morphine in the present study. Additionally, intrathecal morphine administration was reported to decrease VAS up to postoperative 24 hr and reduce opioid requirement up to postoperative 48 hr.16 To demonstrate the non-inferiority of the analgesic effect of ITM-IVPCA over PCTEA, it was thought that the VAS at 24 hrs after surgery should be compared between both groups. Therefore, we set VAS score at postoperative 24 hr as the primary endpoint.
It is well known that continuous epidural analgesia is superior to opioid IVPCA in relieving postoperative pain in patients undergoing abdominal surgery6 including gastrectomy.18 Also, ITM-IVPCA has been reported to reduce postoperative opioid consumption and improve analgesic effect over IVPCA alone.1,19 However, until now, only a few comparative studies between ITM and thoracic epidural analgesia (TEA) have been undertaken. De Pietri, et al.17 demonstrated that, in hepatectomies, ITM-IVPCA provided comparable pain relief to continuous epidural analgesia up to 48 hours after surgery and ITM-IVPCA was proven as a valid alternative to epidural analgesia. However, they revealed that the EP group took a longer time to require first analgesics and consumed less morphine IVPCA, while postoperative nausea and vomiting occurred less frequently, compared to the IT group. These findings are in agreement with our study, which demonstrated that the EP group required less additional fentanyl and less postoperative nausea occurred. Therefore, continuous epidural analgesia seems to have advantages over ITM-IVPCA with regards to postoperative opioid consumption and adverse effects in patients undergoing conventional open abdominal surgery. However, De Pietri, et al.17 unfortunately did not evaluate postoperative complications or outcomes.
As the secondary endpoint, we demonstrated that PCTEA is associated with the reduced incidence of postoperative ileus and pulmonary complication after gastrectomy. Postoperative ileus is a major gastrointestinal complication of abdominal surgery, leading to increased rates of morbidity and mortality, longer lengths of hospital stay, and higher costs.20 Gastrointestinal hypomotility, caused by surgical reflex via inflammatory cascades, leads to postoperative ileus. TEA could increase gastrointestinal activity and improve postoperative ileus without increasing the risk of anastomotic leakage.21 Additionally, there is evidence that TEA preserves pulmonary function better than other analgesic techniques.22,23,24 The decreased incidence of pulmonary complications in the PCTEA group in this study may have resulted from better preservation of pulmonary function by the PCTEA than by the ITM-IVPCA.
Conversely, Levy, et al.5 reported that epidural analgesia in patients undergoing laparoscopic colorectal surgery increased the time to return bowel function and showed similar pulmonary function compared to spinal analgesia in their prospective randomized controlled trial. These differences from our results may be because of a characteristic of the colorectal surgery or the analgesic regimen. However, in their study, the epidural analgesia group received more intravenous fluid than the spinal analgesia group throughout the peri-operative period and had a small degree of motor blockade, which could inhibit early mobility. Excessive fluid administration could lead to edematous bowel and lungs, which consequently causes ileus and adverse respiratory function.25 Immobility can also prevent enhanced recovery.26 In other retrospective investigations in which ITM shortened hospital stay compared with epidural analgesia, epidural analgesia was also associated with a larger amount of intravascular fluid administration or limited ambulation.3,4 However, our results show that fluid overload and motor blockade during epidural analgesia are avoidable. Therefore, when attempting to elucidate the effects of analgesic techniques on postoperative complications or outcomes, one should consider the influences of fluid administration and preservation of ambulation.
This study had several limitations. First, according to the allocated group, participants in our study received different kinds of analgesic medication (morphine and fentanyl vs. ropivacaine and fentanyl) via different routes (intrathecal route combined with intravenous route vs. epidural route). Because 0.3 mg of intrathecal morphine combined with 20 µg/kg of fentanyl in IVPCA has not been proven to have potentially equivalent analgesia to 1000 µg of fentanyl mixed with 0.2% ropivacaine in PCTEA, adopted doses and types of opioids and local anesthetic in our study may affect our results. However, the objective of our study was to compare the two analgesic methods, not to find the appropriate doses or types of analgesic agent for each analgesic method. Therefore, we selected conventional doses of the medications for each analgesic method. Previous studies that compared the analgesic efficacy between different analgesic methods also did not consider equivalent doses for analgesic effect between different medications, but adopted conventional types and doses of analgesic medication for each analgesic method.17,23 Second, in spite of lower incidences of postoperative ileus and pulmonary complication in the PCTEA group, we could not show that PCTEA shortened hospital stay. This may be because our study does not have sufficient power for secondary endpoints such as postoperative outcome. Finally, we did not perform sham blocks in the both groups. Therefore, the present study is not double blind.
In conclusion, analgesia with ITM-IVPCA is not as effective as that of PCTEA, considering the treatment differences in terms of VAS scores failed to demonstrate the non-inferiority of ITM-IVPCA compared to PCTEA. Furthermore, ITM-IVPCA required the higher amount of opioids compared to PCTEA. Moreover, ITM-IVPCA was associated with a longer time to ambulation, an increased incidence of postoperative ileus and pulmonary complication. Therefore, ITM-IVPCA may not be preferred analgesic technique to PCTEA in patients undergoing conventional open gastrectomy.
Figures and Tables
 | Fig. 1CONSORT diagram showing the flow of participants through each stage of our randomized trial. EP, patient-controlled thoracic epidural analgesia; IT, intrathecal morphine combined with intravenous patient-controlled analgesia; CONSORT, consolidated standards of reporting trials. |
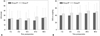 | Fig. 2VAS scores of maximum pain experienced since last questioning (or recovery from anesthesia) (A) at rest and (B) on coughing. The VAS scores were compared using unpaired Student's t-tests. VAS, visual analogue scale; EP, patient-controlled thoracic epidural analgesia; IT, intrathecal morphine combined with intravenous patient-controlled analgesia. |
 | Fig. 3Mean differences in VAS scores of maximum pain experienced at 24 hours after surgery. Error bars indicate two-sided 95% confidence intervals (CIs). As the CIs include Δ and zero, the difference was non-significant, and the results regarding non-inferiority were inconclusive. Δ, margin of non-inferiority. Non-tinged area indicates zone of inferiority. ITM-IVPCA, intrathecal morphine combined with intravenous patient-controlled analgesia; VAS, visual analogue scale; PCTEA, patient-controlled thoracic epidural analgesia. |
Table 1
Patient Characteristics and Data from the Perioperative Period
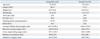
IT, intrathecal morphine combined with intravenous patient-controlled analgesia; ASA, American society of anesthesiologists; CCI, calculated co-morbidities index; RSTG, radical subtotal gastrectomy; RTG, radical total gastrectomy; EP, patient-controlled thoracic epidural analgesia.
Data were shown as mean±SD or number of patients.
References
1. Devys JM, Mora A, Plaud B, Jayr C, Laplanche A, Raynard B, et al. Intrathecal + PCA morphine improves analgesia during the first 24 hr after major abdominal surgery compared to PCA alone. Can J Anaesth. 2003; 50:355–361.

2. Beaussier M, Weickmans H, Parc Y, Delpierre E, Camus Y, Funck-Brentano C, et al. Postoperative analgesia and recovery course after major colorectal surgery in elderly patients: a randomized comparison between intrathecal morphine and intravenous PCA morphine. Reg Anesth Pain Med. 2006; 31:531–538.

3. Sakowska M, Docherty E, Linscott D, Connor S. A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg. 2009; 33:1802–1808.

4. Virlos I, Clements D, Beynon J, Ratnalikar V, Khot U. Short-term outcomes with intrathecal versus epidural analgesia in laparoscopic colorectal surgery. Br J Surg. 2010; 97:1401–1406.

5. Levy BF, Scott MJ, Fawcett W, Fry C, Rockall TA. Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg. 2011; 98:1068–1078.

6. Werawatganon T, Charuluxananan S. WITHDRAWN: Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev. 2013; 3:CD004088.
7. Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000; 321:1493.

8. Rigg JR, Jamrozik K, Myles PS, Silbert BS, Peyton PJ, Parsons RW, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002; 359:1276–1282.

9. Manion SC, Brennan TJ. Thoracic epidural analgesia and acute pain management. Anesthesiology. 2011; 115:181–188.

10. Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004; 101:950–959.

11. Oh SJ, Choi WB, Song J, Hyung WJ, Choi SH, Noh SH, et al. Complications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single institute. J Gastrointest Surg. 2009; 13:239–245.

12. Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Ann Emerg Med. 2001; 37:28–31.

13. Bodian CA, Freedman G, Hossain S, Eisenkraft JB, Beilin Y. The visual analog scale for pain: clinical significance in postoperative patients. Anesthesiology. 2001; 95:1356–1361.
14. Komatsu H, Matsumoto S, Mitsuhata H, Abe K, Toriyabe S. Comparison of patient-controlled epidural analgesia with and without background infusion after gastrectomy. Anesth Analg. 1998; 87:907–910.

15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–383.

16. Meylan N, Elia N, Lysakowski C, Tramèr MR. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. Br J Anaesth. 2009; 102:156–167.

17. De Pietri L, Siniscalchi A, Reggiani A, Masetti M, Begliomini B, Gazzi M, et al. The use of intrathecal morphine for postoperative pain relief after liver resection: a comparison with epidural analgesia. Anesth Analg. 2006; 102:1157–1163.

18. Zhu Z, Wang C, Xu C, Cai Q. Influence of patient-controlled epidural analgesia versus patient-controlled intravenous analgesia on postoperative pain control and recovery after gastrectomy for gastric cancer: a prospective randomized trial. Gastric Cancer. 2013; 16:193–200.

19. Boonmak S, Boonmak P, Bunsaengjaroen P, Srichaipanha S, Thincheelong V. Comparison of intrathecal morphine plus PCA and PCA alone for post-operative analgesia after kidney surgery. J Med Assoc Thai. 2007; 90:1143–1149.
21. Holte K, Kehlet H. Epidural analgesia and risk of anastomotic leakage. Reg Anesth Pain Med. 2001; 26:111–117.

22. van Lier F, van der Geest PJ, Hoeks SE, van Gestel YR, Hol JW, Sin DD, et al. Epidural analgesia is associated with improved health outcomes of surgical patients with chronic obstructive pulmonary disease. Anesthesiology. 2011; 115:315–321.

23. Meierhenrich R, Hock D, Kühn S, Baltes E, Muehling B, Muche R, et al. Analgesia and pulmonary function after lung surgery: is a single intercostal nerve block plus patient-controlled intravenous morphine as effective as patient-controlled epidural anaesthesia? A randomized non-inferiority clinical trial. Br J Anaesth. 2011; 106:580–589.

24. Bauer C, Hentz JG, Ducrocq X, Meyer N, Oswald-Mammosser M, Steib A, et al. Lung function after lobectomy: a randomized, double-blinded trial comparing thoracic epidural ropivacaine/sufentanil and intravenous morphine for patient-controlled analgesia. Anesth Analg. 2007; 105:238–244.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download