Abstract
Purpose
To evaluate whether intraoperative neurophysiologic monitoring (IONM) with combined muscle motor evoked potentials (mMEPs) and somatosensory evoked potentials is useful for more aggressive and safe resection in intramedullary spinal cord tumour (IMSCT) surgery.
Materials and Methods
We reviewed data from consecutive patients who underwent surgery for IMSCT between 1998 and April 2012. The patients were divided into two groups based on whether or not IONM was applied. In the monitored group, the procedures were performed under IONM using 75% muscle amplitude decline weaning criteria. The control group was comprised of patients who underwent IMSCT surgery without IONM. The primary outcome was the rate of gross total excision of the tumour on magnetic resonance imaging at one week after surgery. The secondary outcome was the neurologic outcome based on the McCormick Grade scale.
Results
The two groups had similar demographics. The total gross removal tended to increase when intraoperative neurophysiologic monitoring was used, but this tendency did not reach statistical significance (76% versus 58%; univariate analysis, p=0.049; multivariate regression model, p=0.119). The serial McCormick scale score was similar between the two groups (based on repeated measure ANOVA).
Conclusion
Our study evaluated combined IONM of trans-cranial electrical (Tce)-mMEPs and SEPs for IMSCT. During IMSCT surgery, combined Tce-mMEPs and SEPs using 75% muscle amplitude weaning criteria did not result in significant improvement in the rate of gross total excision of the tumour or neurologic outcome.
Intramedullary spinal cord tumours (IMSCT) are rare neoplasms that account for only 2-4% of central nervous system tumours and 15% of all primary intradural tumours in adults.1,2 Surgery is the most effective treatment for the majority of intramedullary tumours including most ependymomas and haemangioblastomas, although whether aggressive surgical resection of astrocytomas is beneficial remains controversial.1,2,3 Most intramedullary spinal cord neoplasms are low-grade lesions and are well circumscribed. Thus, gross total tumour resection can lead to long-term survival by controlling long-term tumour recurrence and by preserving neurological function.4,5,6 Intraoperative neurophysiologic monitoring (IONM) is one of the modalities that assist in the goal of maximising tumour resection and minimising neurological morbidity.7,8,9,10 Since the application of somatosensory evoked potential (SSEP) in scoliosis surgery was reported in 1977, trans-cranial electronic motor-evoked potentials (MEPs), electromyography (EMG), and direct wave monitoring (D-wave) have been introduced and used in IMSCT surgery.8,11,12,13 The types of IONM modalities or weaning criteria that provide the most reliable results for IMSCT surgery remain unclear. Recently, the combined D-wave and muscle MEPs (mMEPs) monitoring generated notable results. However, combined mMEPs and SSEP monitoring without D-wave has rarely been reported upon for IMSCT surgery.8,9,14,15,16
The aim of our study was to determine whether IONM with combined mMEP using an amplitude of 75% weaning criteria and SSEP is useful for more aggressive and safer resections for IMSCT surgery.
We reviewed consecutive data such as clinical charts, operative notes, neurophysiologic data, and neuro-imaging studies from patients who underwent surgery for IMSCT between 1998 and April 2012. The patients were divided into two groups based on whether IONM was applied or not. The monitored group (Group A) consisted of patients who underwent IMSCT surgery using trans-cranial electrical (Tce)-mMEP/SEP IONM between July 2006 and April 2012. The control group (Group B) consisted of patients who underwent IMSCT surgery without IONM between 1998 and July 2006. All operations were performed by one senior spine neurosurgeon (S.C.R.) within a single institute. Our study was conducted with the approval of the Institutional Review Board (local institute IRB number S2012-1952-0001).
We compared demographic factors between the two groups including sex, age, body mass index (BMI), location of the tumour, the tumour size, combination with syrinx, and histological diagnosis. For sub-category analyses, we divided the histological diagnosis into 5 categories, such as astrocytoma, ependymoma, haemangioblastoma, cavernous haemangioma, and others. We also divided the patients based on the lesion site: cervical, thoracic, and lumbar. The size of the tumour was calculated based on the maximum diameter of the lesion.
Anaesthesia was maintained with continuous infusion of propofol (10 mg/kg/h) and remifentanil (0.25 mg/kg/min). A single bolus of non-depolarising short-acting muscle relaxant (rocuronium) was administered at induction to facilitate tracheal intubation and ventilation.
No paralytic agents were used after induction and intubation. The level of neuromuscular block was monitored by recording the compound muscle action potentials to a train of four stimuli. Invasive blood pressure, electrocardiogram, end-tidal carbon dioxide concentration, pulse oximetry, and temperature were all monitored. The bispectral index was used for monitoring the depth of sedation.17
Stimulation of SEPs was accomplished using square wave electrical pulses of 0.3 ms in duration and a maximum intensity of 25 mA at a frequency of 4.7 Hz. Surface-stimulating electrodes were placed over each median nerve at the wrist and over each posterior tibial nerve at the ankle. Evoked potentials were recorded in a referential fashion from the C3' (2 cm posterior to C3, right median nerve stimulation), C4' (2 cm posterior to C4, left median nerve stimulation), and Cz' (2 cm posterior to Cz, right and left tibial nerve stimulation) positions and from a reference electrode at FPZ (international 10-20 system). The low-filter setting was typically 30 Hz, but in some cases, 50 Hz was used. The high-filter setting was 3000 Hz.
Multi-pulse transcranial electrical stimulation was performed using a commercially available intraoperative monitoring (IOM) electrical stimulator (NeuropackMEB-9200K; Nihon Kohden Co., Tokyo, Japan). Nine-millimetre disc electrodes were attached to the scalp using colloid ion 6 cm anterior to Cz and at C3 and C4 (international 10-20 system). trains of 6 pulses (individual stimulus duration: 50 ms) with inter-stimulus intervals of 3 ms were used with a period of at least 30 seconds between two successive trains. Stimulus intensity was increased gradually (50 V increments from 100 V to a maximum of 600 V) until MEP amplitudes were maximised above a minimum of 20 mV. If response amplitudes of at least 20 mV were not obtained from either leg, MEP monitoring was abandoned. MEPs were recorded simultaneously from the tibialis anterior and abductor hallucis muscles of both legs and from the abductor pollicis muscles of both arms using a pair of non-insulated subcutaneous needle electrodes inserted 3 cm apart in each muscle. The time base was 100 ms. The typical low-filter setting used was 30 Hz, but in some cases, 50 Hz was used. The high-filter setting used was 3000 Hz.
A drop in amplitude of 50% or a prolongation in SSEP latency of 10% was considered a significant finding. If meaningful SEP changes occurred prior to myelotomy, the procedure was stopped temporarily and the corresponding the change was addressed. However, if the SSEP change occurred during the myelotomy, the myelotomy continued regardless of the SSEP finding.
A drop in mMEP of 75% or complete loss from baseline amplitude was considered a significant finding. If any significant changes in mMEP occurred, the surgical procedure was stopped temporarily. We then checked the mean blood pressure (BP) and increased the BP to at least above 60 mm Hg. Additionally, we irrigated the surgical field using warm saline solution. If possible, we temporally skipped the site causing the alarm. We performed procedures at other tumour marginal sites. After all corrective methods, the surgery withdraw in selected cases regarding tumour histological findings, the patient's preoperative neurological status.
We monitored MEP and SEP changes regularly, particularly during the following steps: 1) positioning the patient, 2) laminectomy, 3) dura opening, 4) tumour resection, 5) dura closure, 6) after laminar insertion. In addition, we performed MEP whenever the surgeon checked for spinal cord injury.
The primary outcome was the rate of gross total excision of the tumour. We evaluated postoperative magnetic resonance images for total excision at 1 week after the surgery. The neuro-radiologist confirmed tumour excision and residual tumour. The secondary outcome was neurologic outcome. We used the McCormick Grade scale to assess the neurologic outcome.2 Patients were graded a total of three times: upon admission, at postoperative week 1, and during the outpatient clinic stage. Neurologic examinations were conducted by a neurosurgery or rehabilitation medicine resident during the immediate postoperative week (week 1). The follow-up assessments were conducted by attending neurosurgeons during clinic visits.
Data were imported into SPSS (version 20.1; SPSS Inc., Chicago, IL, USA) for analysis. Based on the characteristics of the variables, we used unpaired Student's t-tests or chi-squared tests to determine significant differences between the two groups in sex, age, body mass index, location of tumour, tumour size (max, diameter), syrinx combined or not, and histological diagnoses. We performed repeated measure ANOVA to compare the effect of monitoring in real time for IMSCT surgery and to compare outcomes based on the McCormick scale during the preoperative period, the immediate post-op (one week) period, and during the outpatient clinic stage after surgery. Uni-variate regression analyses were performed to assess the individual effects of sex, age, tumour size, tumour location, and histological diagnosis on the rate of total excision. A stepwise multiple linear regression was then performed using backward elimination to select an appropriate model. The p value >0.10 was used for removal.
We enrolled a total of 76 patients who underwent surgery for IMSCT between 1998 and April 2012. The monitored group consisted of 50 patients who underwent IMSCT surgery with IONM between July 2006 and April 2012. The control group included 26 patients operated on between 1998 and July 2006. The mean follow-up duration was 18.2 months in the monitored group and 31.3 months in the unmonitored group.
Baseline characteristics are compared in Table 1. Overall, both cohorts were similar. The mean age of the study population was 42.7 years in the monitored group and 40.1 years in the non-monitored group. In the monitored group, the tumour size was slightly smaller than in the unmonitored group. Combined syrinx was observed more frequently in the monitored group. With respect to histological diagnoses, the rate of ependymoma was 44% and 42% in the monitored and non-monitored group, respectively. In the unmonitored group, patients with astrocytoma were more likely to enroll (14% versus 27%) and the number of patients with haemangioblastoma was lower (26% versus 15%), but there were no statistically significant differences between the groups. There was no statistically significant difference in sex, age, BMI, location of tumour, tumour size, combination with syrinx, or histological diagnoses.
In the IONM group, the gross total tumour excision rate was greater than in the non-IONM group, but there was no significant difference (76% versus 58%; univariate analysis, p=0.049; multivariate regression model, p=0.119). We first performed univariate regression analyses to assess the factors affecting gross total excision. The female sex, tumour size, combination with syrinx, histological diagnosis, and being monitored showed an association with gross total excision (Table 2). In the final multiple linear regression model (Table 3), we built a multivariate logistic model to adjust for confounding factors. In the final model, the histological diagnosis was the only variable that was significantly associated with gross total resection. The use of monitoring did not result in a statistically significant effect [odds ratio (OR): 3.14, 95% confidence interval (CI), 0.75-13.33, p-value=0.119].
The neurologic outcome based on the McCormick scale score was not significantly different between the two groups. At the preoperative baseline, both groups showed a similar McCormick scale score (1.64 versus 2.07, p=0.083). After surgery, the outcome score worsened during the immediate postoperative stage (2.10 versus 2.65, p=0.076). During the follow-up period, the scores tended to improve (1.82 versus 2.31, p=0.115). Over time, the McCormick Grade score of each group showed a tendency to worsen during the immediate post-operative stage compared with the preoperative stage and a tendency to improve during the outpatient clinic stage compared with the immediate post-operative period (Table 4, Fig. 1). Moreover, when histological diagnosis (astrocytoma, ependymoma, haemangioblastoma) was included, this trend was maintained (Table 5, Fig. 2).
The sensitivity and specificity of predicting postoperative neurologic deficit was 94% (17/18) and 94% (30/32). The positive predictive value and negative predictive value were 89% (17/19) and 97% (30/31). Within the 50 cases, false positives (2 cases) and a false negative (1 case) were observed. The false negative case was a 67-year-old man with ependymoma from C7 to T1. IONM revealed mMEP amplitude changes between 50% and 75%, but this change did not satisfy our criteria. After surgery, the patient showed paraparesis (Gr. 4), but at 6 months, his condition had improved (Gr. 4→5).
IONM for safe spine surgery of scoliosis was first reported in 1977. SSEP, MEP and EMG have since been introduced and applied for correction of deformities or insertion of pedicle screws.11,12 However, intramedullary spinal cord tumours are rare, and for years, reports on the use of IOM techniques during their surgical removal have remained anecdotal. Before the advent of MEP during IMSCT surgery, SEPs were used alone with the assumption that changes in SEPs specifically represented spinal cord dysfunction.18 False negative results occurred during surgeries monitored only with SEPs. The patient woke up with a new paraparesis that would not have been recognised if the patient were using SEPs alone.19 The proposal to have neurophysiologic parameters as a major outcome predictor of spinal cord surgery emerged in 1997 when Morota, et al.14 introduced the use of D-wave (epidural MEP) monitoring after transcranial electrical stimulation and concluded that this method appeared to be a better predictor of functional outcome than the patient's pre-operative motor status. Since Kothbauer, et al.8 used a combination of D-wave and mMEPs for 100 patients undergoing IMSCT surgery, there have been several reports claiming aggressive tumour mass removal and preservation of normal neurologic function. Finally, in 2005, Sala, et al.9 concluded that the applied motor evoked potential method appeared to significantly improve long-term motor outcome. Similarly, early motor outcome due to transient motor deficits in the monitored group can be predicted by the neurophysiologic profile of the patient at the end of surgery. In that report, a combined D-wave and mMEP monitoring protocol was used. D-wave and mMEPs form a complementary relationship. The IONM modality for IMSCT surgery may be divided into those with or without D-wave.
Various modalities have been adopted for IMSCT without D-wave. These modalities include threshold-level parameters during multi-pulse transcranial electrical stimulation (TES), mMEP morphology (from polyphasic to biphasic to loss) and free-running EMG combined with mMEP monitoring.20,21,22,23
In our study, all patients were operated on using combined mMEP and SSEP monitoring. Particularly, we used a 75% mMEP amplitude criterion because we believed a 100% decline in amplitude to be too specific and a 50% decline in amplitude to be too sensitive.24,25,26,27 If the monitoring alarm is too sensitive, the surgery is stopped prematurely. If the alarm is too specific, there is risk of damaging the normal spinal cord.
Combining mMEP and D-wave monitoring may be the gold standard for IMSCT surgery. We could not use D-wave monitoring due to a holding provision with our local insurance. Our protocol choice was a combination of mMEP and SEP monitoring without D-wave, a unique IONM protocol for IMSCT.
The effect of various modalities for IMSCT surgery remains unclear.21,27,28,29 In our study, all surgical intervention was performed if the mMEP amplitude was less than 75% of baseline MEP amplitude. If the surgery was abandoned due to a change in amplitude of 50%, such termination of surgery precludes a complete resection of the tumour. In contrast, if the surgery is stopped based on an 'absence-presence criterion', a high rate of "false positives" may occur. False positives result in incomplete tumour mass removal. Conversely, the false negative is disastrous for both surgeon and patient. Although the tumour is completely removed, the patient will sustain severe neurologic deficits. Thus, it is important to determine a reasonable compromise. No report has shown whether mMEP amplitude criteria are reliable. We suggest that a 75% criterion is the most reliable criterion. Thus, we verified the specificity, sensitivity, positive predictive value, negative predictive value, false positive rate, and false negative rate (Table 6).
There have been various reports discussing IONM with gross total tumour excision. The majority reported a negative effect of IONM on gross total tumour mass removal. Surgeries were frequently stopped too early because the IONM was too sensitive.9,22,26 In our study, gross total resection (GTR) was superior to not monitoring cases. Although it failed to exert a statistically significant effect in the final adjusted model, IONM tended to enable complete tumour mass removal. We believe that there are several reasons that may underlie the slight increase in total tumour mass removal in the monitored group. 1) The surgeon believed that IONM enabled a safer surgery, a vitally important point for the surgeon. A stable IONM enabled the surgeon to be more aggressive during tumour mass resection. IONM encouraged the surgeon to proceed with tumour mass resection. 2) IONM not only prevented neural injury but also provided opportunities to correct reversible injury by intervention. 3) Although not reaching statistical significance in the monitored group, the number of haemangioblastoma patients was slightly greater than the number of patients with astrocytoma. Haemangioblastoma can be more easily removed than astrocytoma. This last point is very difficult to assess due to the surgeon's learning curve. Thus, we considered the learning curve and excluded 10 years of operations prior to 1998. Nevertheless, this point may cause some bias.
Our study had several important limitations. This study was a retrospective analysis; we could not design prospective or randomised controlled studies due to ethics. Without prospective studies that are powered to detect differences, it may not be possible to determine the potential value of neurological monitoring in these cases. The follow-up time was different between the two groups. Although we included cases that were observed for at least 1 year, the follow-up time differed by 13 months. The number of patients in the control group was relatively smaller than in the monitored group. A greater number in the control group will provide power to detect the mean difference between groups.
Our data demonstrates the use of combined IONM using Tce-mMEP and SEP for IMSCT. During IMSCT surgery, combined Tce-mMEP and SEP, using a 75% muscle amplitude weaning criterion, did not cause a significant improvement in the rate of gross total excision of the tumour or neurologic outcome. IONM for IMSCT does not reduce the total tumour excision rate, although there had been concerns that IONM during IMSCT blocked the surgical procedure and resulted in incomplete tumour mass removal.
Figures and Tables
 | Fig. 2Repeated measured ANOVA comparing effect of monitoring according IMSCT histology. POD, post operative day; IMSCT, intramedullary spinal cord tumour; ANOVA, analysis of variance. |
Table 1
Baseline Demographic Characteristics, Following Clinical and Radiologic Findings (Group A: Monitored, Group B: Non-Monitored)
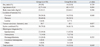
Table 2
Univariate Analysis Showing the Individual Effects of Sex, Age, Tumour Size, Tumour Location, Histological Diagnosis, Monitoring on Total Excision Rate
ACKNOWLEDGEMENTS
We appreciate critical advice from Dr. Seok-Ho Hong and correction of the formatting by YM Jeong.
References
1. Cristante L, Herrmann HD. Surgical management of intramedullary spinal cord tumors: functional outcome and sources of morbidity. Neurosurgery. 1994; 35:69–74.
2. McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990; 72:523–532.

3. Hoshimaru M, Koyama T, Hashimoto N, Kikuchi H. Results of microsurgical treatment for intramedullary spinal cord ependymomas: analysis of 36 cases. Neurosurgery. 1999; 44:264–269.

4. Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000; 93:2 Suppl. 183–193.

5. Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg. 1993; 79:204–209.

6. Guidetti B, Mercuri S, Vagnozzi R. Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg. 1981; 54:323–330.

7. Ney JP, van der Goes DN. Evidence-based guideline update: Intraoperative spinal monitoring with somatosensory and transcranial electrical motor evoked potentials. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology. 2012; 79:292.

8. Kothbauer KF, Deletis V, Epstein FJ. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg Focus. 1998; 4:e1.

9. Sala F, Palandri G, Basso E, Lanteri P, Deletis V, Faccioli F, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006; 58:1129–1143.

10. Nuwer MR, Emerson RG, Galloway G, Legatt AD, Lopez J, Minahan R, et al. Evidence-based guideline update: intraoperative spinal monitoring with somatosensory and transcranial electrical motor evoked potentials: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology. 2012; 78:585–589.

11. Nash CL Jr, Lorig RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop Relat Res. 1977; 100–105.

12. Calancie B, Madsen P, Lebwohl N. Stimulus-evoked EMG monitoring during transpedicular lumbosacral spine instrumentation Initial clinical results. Spine (Phila Pa 1976). 1994; 19:2780–2786.
13. Danesh-Clough T, Taylor P, Hodgson B, Walton M. The use of evoked EMG in detecting misplaced thoracolumbar pedicle screws. Spine (Phila Pa 1976). 2001; 26:1313–1316.

14. Morota N, Deletis V, Constantini S, Kofler M, Cohen H, Epstein FJ. The role of motor evoked potentials during surgery for intramedullary spinal cord tumors. Neurosurgery. 1997; 41:1327–1336.

15. Sala F, Beltramello A, Gerosa M. Neuroprotective role of neurophysiological monitoring during endovascular procedures in the brain and spinal cord. Neurophysiol Clin. 2007; 37:415–421.

16. Deletis V. Intraoperative neurophysiology of the corticospinal tract of the spinal cord. Suppl Clin Neurophysiol. 2006; 59:107–112.
17. Hyun SJ, Rhim SC. Combined motor and somatosensory evoked potential monitoring for intramedullary spinal cord tumor surgery: correlation of clinical and neurophysiological data in 17 consecutive procedures. Br J Neurosurg. 2009; 23:393–400.

18. Kearse LA Jr, Lopez-Bresnahan M, McPeck K, Tambe V. Loss of somatosensory evoked potentials during intramedullary spinal cord surgery predicts postoperative neurologic deficits in motor function [corrected]. J Clin Anesth. 1993; 5:392–398.

19. Ginsburg HH, Shetter AG, Raudzens PA. Postoperative paraplegia with preserved intraoperative somatosensory evoked potentials. Case report. J Neurosurg. 1985; 63:296–300.

20. Quiñones-Hinojosa A, Lyon R, Zada G, Lamborn KR, Gupta N, Parsa AT, et al. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005; 56:982–993.
21. Calancie B, Harris W, Brindle GF, Green BA, Landy HJ. Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg. 2001; 95:2 Suppl. 161–168.

22. Skinner SA, Nagib M, Bergman TA, Maxwell RE, Msangi G. The initial use of free-running electromyography to detect early motor tract injury during resection of intramedullary spinal cord lesions. Neurosurgery. 2005; 56:2 Suppl. 299–314.

23. Hyun SJ, Rhim SC, Kang JK, Hong SH, Park BR. Combined motor-and somatosensory-evoked potential monitoring for spine and spinal cord surgery: correlation of clinical and neurophysiological data in 85 consecutive procedures. Spinal Cord. 2009; 47:616–622.

24. Jones SJ, Harrison R, Koh KF, Mendoza N, Crockard HA. Motor evoked potential monitoring during spinal surgery: responses of distal limb muscles to transcranial cortical stimulation with pulse trains. Electroencephalogr Clin Neurophysiol. 1996; 100:375–383.

25. Sutter M, Eggspuehler A, Grob D, Jeszenszky D, Benini A, Porchet F, et al. The validity of multimodal intraoperative monitoring (MIOM) in surgery of 109 spine and spinal cord tumors. Eur Spine J. 2007; 16:Suppl 2. S197–S208.

26. Albright AL. Intraoperative spinal cord monitoring for intramedullary surgery: an essential adjunct. Pediatr Neurosurg. 1998; 29:112.

27. Calancie B, Molano MR. Alarm criteria for motor-evoked potentials: what's wrong with the "presence-or-absence" approach? Spine (Phila Pa 1976). 2008; 33:406–414.
28. Fehlings MG, Brodke DS, Norvell DC, Dettori JR. The evidence for intraoperative neurophysiological monitoring in spine surgery: does it make a difference? Spine (Phila Pa 1976). 2010; 35:9 Suppl. S37–S46.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download