Abstract
Purpose
Relatively little is known on the microbiology, risk factors and outcomes of peritoneal dialysis (PD)-associated peritonitis in Korean children. We performed this study in order to evaluate the incidence, treatment and clinical outcomes of peritonitis in pediatric PD patients at Severance Hospital.
Materials and Methods
We analyzed data from 57 PD patients younger than 18 years during the period between June 1, 1986 and December 31, 2011. The collected data included gender, age at commencement of PD, age at peritonitis, incidence of peritonitis, underlying causes of end stage renal disease, microbiology of peritonitis episodes, antibiotics sensitivity, modality and outcomes of PD.
Results
We found 56 episodes of peritonitis in 23 of the 57 PD patients (0.43 episodes/patient-year). Gram-positive bacteria were the most commonly isolated organisms (40 episodes, 71.4%). Peritonitis developed in 17 patients during the first 6 months following initiation of PD (73.9%). Peritonitis episodes rarely resulted in relapse or the need for permanent hemodialysis and no patient deaths were directly attributable to peritonitis. Antibiotic regimens included cefazolin+tobramycin from the years of 1986 to 2000 and cefazolin+ceftazidime from the years of 2001 to 2011. While antibiotic therapy was successful in 48 episodes (85.7%), the treatment was ineffective in 8 episodes (14.3%). The rate of continuous ambulatory PD (CAPD) peritonitis was statistically higher than that of automated PD (APD) (p=0.025).
Peritoneal dialysis (PD) is the preferred dialysis modality for children requiring renal replacement therapy and peritonitis is a common complication of PD. PD-associated peritonitis occurs more frequently in children compared to adults, and remains an important cause of morbidity, mortality and irreversible technique failure therein.1-4 The International Pediatric Peritonitis Registry (IPPR) previously reported on the outcomes of 548 episodes of PD peritonitis in 392 pediatric patients from 44 pediatric dialysis centers, and the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) found 0.68 peritonitis episodes per patient-year in PD-treated children.1,3,4
The organisms that cause peritonitis and their antibiotic sensitivities are known to vary by region. Accordingly, the International Society of Peritoneal Dialysis (ISPD) recommended the use of antibiotics according to the known conditions of each region.5 However, to date, there have been relatively few published articles on the microbiology, risk factors and outcomes of PD-associated peritonitis in Korean children.6-8 The purpose of this study was to assess the incidence, predictors, treatment and clinical outcomes of peritonitis in Korean pediatric PD patients at a single center over the last 25 years.
Data from all children who had received PD at Severance Hospital in Seoul, South Korea between June 1, 1986 (when detailed peritonitis data were available) and December 31, 2011 were reviewed retrospectively. Children were younger than 18 years of age at the commencement of PD.
In all patients, a double-cuff Tenckhoff straight catheter was surgically placed utilizing the downward-facing tunnel method. A prophylactic antibiotic (cefazolin) was administered 2 hours before the operation, after which subcutaneous tunneling was performed. Dialysate comprised 1.5%, 2.5% and 4.25% dextrose PD fluid, and was selected according to the volume status of the patients. Dialysate was exchanged performing the manual exchange technique in continuous ambulatory PD (CAPD); 1.5% dialysate was exchanged 3 times, and 2.5% or 4.25% dialysate was exchanged 1-2 times per day. In automated PD (APD), continuous cyclic PD was performed during the night according to the peritoneal equilibration test, and the Home Choice cycler (Baxter®, Baxter Int., Deerfield, IL, USA) was used.
Microorganisms were isolated from peritoneal fluid and cultured on blood agar plates and MacConkey agar plates for 3 days. Sabraud dextrose agar plate was used for the cultivation of fungi for 4 weeks. During the study period, the methodologies were not changed.
The collected data included information on gender, age at commencement of PD, age at peritonitis, incidence of PD peritonitis, causes of end stage renal disease (ESRD), microbiology of peritonitis episodes, antibiotics sensitivity, initial treatment modality and outcomes. The duration of PD was defined as the time between the commencement date and the cessation date.
Diagnosis of peritonitis was made based on the following three criteria and diagnosed when any two of the following three criteria are present: 1) cloudy peritoneal fluid and abdominal pain; 2) peritoneal fluid containing more than 100 white blood cells/mm3 with at least 50% polymorphonuclear cells; and 3) microorganisms in the peritoneal fluid.5 For the patients with multiple episodes of peritonitis, all episodes were separate episodes.
Microorganisms were isolated in a peritoneal fluid culture study and antibiotic sensitivity was investigated for first generation cephalosporin (cefazolin) and glycopeptide (vancomycin) in gram-positive organisms and 3rd generation cephalosporin (ceftazidime) and tobramycin in gram-negative organisms. The mean duration of antibiotics therapy was 18.5±2.6 days (range 14-23 days) and an antifungal agent was administered in one patient for 4 weeks. Antibiotics were administered by intraperitoneal injection.
The outcomes observed in the study included rates of relapse, recurrent peritonitis, catheter removal, temporary or permanent transfer to hemodialysis and patient death. Peritonitis relapse was defined as an episode of peritonitis due to the same organism occurring within 4 weeks of administration of the last antibiotics, regardless of antibiotics sensitivity.5 Recurrent peritonitis was defined as an episode of peritonitis due to a different organism occurring within 4 weeks of administration of the last antibiotics.5 Peritonitis-related death was recorded if the patient's death was directly attributed to peritonitis based on the clinical opinion of the treating nephrologist.
Results were evaluated using frequencies and percentages for categorical variables and means with standard deviation or median with ranges for continuous variables. Data were analyzed by t-test, chi-square test, Fisher's exact test, Mann-Whitney U test using SPSS software (version 17.0, SPSS Inc., Chicago, IL, USA) for Windows. A p-value of less than 0.05 was considered statistically significant.
A total of 57 patients who were younger than 18 years (40 male, 70.2%; 17 female, 29.8%) were treated with PD during the study period (from June 1, 1986 to December 31, 2011). The mean duration of PD therapy was 27.5±40.5 months (median 11 months, range 1 to 240 months).
Fifty-six episodes of peritonitis occurred in 23 individuals (16 males, 7 females) resulting in 0.43 episodes per patient-year of treatment. The incidence of peritonitis was highest during 1996-2000 and decreased during the subsequent years (Fig. 1). Fifty-one patients received CAPD, while five received APD and one crossover. Peritonitis episodes were significantly lower in children on APD (0.06 episodes/patient-year) compared to those on CAPD (0.48 episodes/patient-year) (p=0.025) (Fig. 2).
In this study, age groups were subdivided as 0-5, 6-10, 11-15 and 16-18 years of age. While the lowest incidence of peritonitis was observed in the 11-15 years age group (0.32 episodes/patient-year), there were no statistically significant differences in the incidence of peritonitis among these groups (p=0.41) (Fig. 3).
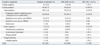
Assessing the etiology of ESRD in our study, the most common causes of ESRD were chronic glomerulonephritis (50 patients, 87.7%), such as focal segmental glomerulosclerosis (31 patients, 54.4%), lupus nephritis (2 patients, 3.5%), rapidly progressive glomerulonephritis (12 patients, 21.0%) and IgA nephropathy (5 patients, 8.8%), and, urological abnormality, such as vesicoureteral reflux and dysplastic kidney (5 patients, 8.8%) and neuroblastoma (2 patients, 3.5%). The mean age at the onset of peritonitis was 11.3±4.5 years with a range of 0.5 to 18 years and the average duration from the initiation of PD to the onset of the first episode of peritonitis was 5.7±11.5 months.
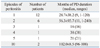
Twelve patients (52.2%) experienced one episode of peritonitis during the study period and 11 (47.8%) experienced more than two episodes of peritonitis (Table 1 and 2). There were no differences between gender or the total duration of PD between children with peritonitis and children without. The age of PD commencement did not differ in patients with peritonitis compared to those without (p=0.81). In patients with more than two episodes of peritonitis, the age of PD commencement was younger and the total duration of PD was longer compared to those with one episode of peritonitis, but these differences were not statistically significant (Table 1).
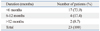
Peritonitis occurred within the first 6 months of PD initiation in 17 patients (73.9%), between 6 and 12 months in 4 patients (17.4%) and after more than 12 months in 2 patients (8.7%) (Table 3). The mean duration between the first and second episode of peritonitis was significantly shorter compared to the duration between PD commencement and the first episode of peritonitis (3.0±2.3 months vs. 5.8±11.5 months, p=0.03).
No organism was isolated in eight episodes (14.3%) of peritonitis. Among the remaining 48 episodes, gram-positive bacteria accounted for 40 episodes (71.4%) and gram-negative bacteria accounted for 7 (12.5%). A fungal infection occurred in one episode (1.8%). Among the gram-positive organisms, the most commonly isolated organism was coagulase-negative staphylococcus (Staphylococcus epidermidis) (46.4%) and the most commonly isolated gram-negative organism was Pseudomonas aeruginosa (8.9%) (Table 4).
The majority of pediatric PD patients were treated with a first generation cephalosporin (cefazolin) in combination with an aminoglycoside (tobramycin) or a third generation cephalosporin (ceftazidime) as an initial empiric therapy for their first episode of peritonitis.
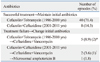
Fourty episodes (71.4%) of peritonitis were treated with cefazolin and tobramycin between 1986 and 2000 and 8 episodes (14.3%) were treated with cefazolin and ceftazidime between 2001 and 2011. Patients were kept on the same regimen if they are sensitive to the cultured organisms or showed a good clinical response to antibiotics. In five episodes (8.9%) of peritonitis, antibiotics were changed from cefazolin and tobramycin to ceftazidime and vancomycin because the initial antibiotics were not clinically effective and were also resistant to the causative organisms according to antibiotic sensitivity. Among these cases, two infections were related to an abdominal tunnel infection. In two episodes (3.6%), antibiotics were changed from cefazolin and ceftazidime to ceftazidime and vancomycin according to the antibiotic sensitivity of the causative organisms. These two patients were infected with methicillin-resistant Staphylococcus aureus (MRSA) upon a culture. In these patients, the PD catheter was removed from the patient and PD was changed to permanent hemodialysis (Table 5). The one episode of fungal peritonitis was treated initially with cefazolin and ceftazidime, but subsequently altered to an antifungal agent (microsomal amphotericin B) based on the cultured organisms.
Moreover, antibiotic sensitivity differed according to age. In the group of children <5 years of age, gram-positive organisms were sensitive to cefazolin (100%), but this sensitivity decreased in the older groups (5-9 years, 66.7%; ≥10 years, 55.0%). In our study, 100% of the gram-positive organisms (n=40) were sensitive to vancomycin regardless of age groups, and in the group of children <5 years of age, the organisms were sensitive to all antibiotics utilized (Table 6).
Most patients were treated initially using empirical antibiotics. Two patients underwent removal of the Tenckhoff catheter because of refractory peritonitis despite antibiotic therapy. MRSA was isolated in both of these patients. The episodes of peritonitis infrequently resulted in both relapse (1.8%) and the need for transition to permanent hemodialysis (1.8%).
PD is the preferred modality for treating children requiring renal replacement therapy due to its simplicity and benefit of preserving residual renal function.9-12 However, peritonitis is a common complication of PD. Known associated factors include catheter exit site infection, tunnel infection and dialysis fluid contamination.1,11 While the incidence of peritonitis in children on PD varies by country, it has decreased due to improved techniques for PD catheter insertion and increased education in parents and patients. In our study, the incidence of peritonitis was highest in the years of 1996-2000 and decreased after 2000.
While recent studies have reported very low rates of peritonitis (0.22-0.26 episodes/patient-year) in children with PD,13,14 peritonitis remains the primary cause of PD failure. The results of our study demonstrated that PD is safe for children requiring renal replacement therapy, and the prevalence of peritonitis in our study was comparable with other large pediatric studies.1,2,15 The rate of peritonitis (0.43 episodes/patient-year) in our study was similar to that reported for children at another hospital in Korea (0.45 episodes/patient-year).6 The rate of PD peritonitis was reported to be 0.68 episodes/patient-year for the year 2007 according to NAPRTCS,16 and 0.71 episodes/patient-year in 2010 in Australia and New Zealand.10
The mean duration between the first and second episode of peritonitis was significantly shorter than that between PD commencement and a first episode of peritonitis (3.0±2.3 months vs. 5.8±11.5 months, p=0.03). This could have resulted because it is easier for infection to occur in patients where peritonitis has occurred once before.
According to the modality of PD, the rate of PD-associated peritonitis was shown to be lower in APD compared to CAPD, and we found a similar result in our study (p=0.025).17-19 The possible reasons for a lower incidence of peritonitis in APD have been previously analyzed and include performing a flush (when compared with CAPD), fewer connections and a potentially positive effect of the 'dry day' after APD.19
In our study, the most common cause of ESRD was chronic glomerulonephritis (87.7%), because our hospital is one of the largest hospitals in Korea and most of our patients comprising congenital anomalies of the kidney and urinary tract patients who received preemptive kidney transplantation as such the majority never go on dialysis. In addition, patients with chronic glomerulonephritis were frequently transferred to our hospital.
IPPR recently reported the outcomes of 548 episodes of PD peritonitis in 392 pediatric patients from 44 pediatric dialysis centers.3,4 The study showed widespread variations in practice, microbiology and outcomes for peritonitis across the world.3,4 In accordance with the current international recommendaguidelines published by IPPR,20 first generation cephalosporin and ceftazidime were recommended to treat PD-associated peritonitis. The ISPD recommended the use of glycopeptide in patients with fever, abdominal pain, MRSA infection history, exit site or tunnel infection and Staphylococcus aureus colonization.20 In the modified ISPD guidelines from 2005, the use of first generation cephalosporin or glycopeptides is recommended according to region and patient characteristics.5
Data from the IPPR suggest that the empiric use of an aminoglycoside instead of a third generation cephalosporin may provide improved treatment against gram-negative organisms in some regions and that local sensitivity patterns are important in determining the initial kind of empiric antibiotic therapy.5 Although the use of aminoglycosides needs careful monitoring to prevent potential ototoxicity, the ISPD concluded that there was no convincing evidence that short-term aminoglycoside therapy adversely affected residual renal function.5
The most commonly noted causative organisms in PD-associated peritonitis are Staphylococcus epidermidis (22.2%) and Staphylococcus aureus (14.6%).21,22 In our study, gram-positive organisms accounted for 71.4% of the total organisms found, with Staphylococcus epidermidis accounting for 46.4%, and Staphylococcus aureus accounting for 23.2%. The number of patients with polymicrobial peritonitis was small (1 episode, 1.8%).
The antibiotic sensitivity to cefazolin for gram-positive organisms was 62.5% and the sensitivity to ceftazidime for gram-negative organisms was 85.7%. While 28.6% of gram-negative organisms were sensitive to tobramycin, 100% were sensitive among children <5 years of age. Based on our results, tobramycin may be useful in the treatment of PD peritonitis in children of less than 5 years of age. Therefore, the use of current empiric antibiotics seems to be appropriate in our hospital.
In our study, all patients were treated on admission and there was no patient cared for at home. There was no patient death directly attributable to peritonitis.
In conclusion, peritonitis remains a common complication of PD therapy in children. Despite a variety of antibiotic regimens that were utilized in this study, we also demonstrated a low peritonitis rate in our center. We concur in conjunction with the IPPR recommendation that local monitoring of infectious organisms and resistance patterns are most appropriate in guiding empiric treatment. Moreover, APD can be considered in selection of PD modality in children. However, further prospective studies with a larger number of patients is required to confirm the results of our study.
Given the risk of relapse or recurrence, further prospective studies are necessary to determine the optimal period of treatment for pediatric patients with PD-related peritonitis. Further studies are also required in order to establish the risk and benefit of topical therapies in order to prevent PD-related peritonitis in children.
Figures and Tables
Fig. 2
Comparison of peritonitis rate between CAPD and APD (total n=57). *Continuous ambulatory peritoneal dialysis. †Automated peritoneal dialysis. CAPD, continuous ambulatory PD; APD, automated PD; PD, peritoneal dialysis.

References
1. Furth SL, Donaldson LA, Sullivan EK, Watkins SL. North American Pediatric Renal Transplant Cooperative Study. Peritoneal dialysis catheter infections and peritonitis in children: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol. 2000; 15:179–182.

2. Kuizon B, Melocoton TL, Holloway M, Ingles S, He-Jing , Fonkalsrud EW, et al. Infectious and catheter-related complications in pediatric patients treated with peritoneal dialysis at a single institution. Pediatr Nephrol. 1995; 9:Suppl. S12–S17.

3. Warady BA, Feneberg R, Verrina E, Flynn JT, Müller-Wiefel DE, Besbas N, et al. Peritonitis in children who receive long-term peritoneal dialysis: a prospective evaluation of therapeutic guidelines. J Am Soc Nephrol. 2007; 18:2172–2179.

4. Zurowska A, Feneberg R, Warady BA, Zimmering M, Monteverde M, Testa S, et al. Gram-negative peritonitis in children undergoing long-term peritoneal dialysis. Am J Kidney Dis. 2008; 51:455–462.

5. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005; 25:107–131.

6. Lee SE, Han KH, Jung YH, Lee HK, Kang HG, Cheong HI, et al. Peritonitis in children undergoing peritoneal dialysis: 10 years' experience in a single center. J Korean Soc Pediatr Nephrol. 2010; 14:174–183.

7. Lee SG, Cho J, Sohn YB, Park SW, Kim SJ, Jin DK, et al. Peritoneal dialysis associated peritonitis and empirical antibiotics therapy in Korean children with chronic renal failure. J Korean Soc Pediatr Nephrol. 2008; 12:213–220.

8. Koo JW, Ha TS, Lim IS, Ha IS, Cheong HI, Choi Y, et al. Peritonitis during CAPD in children. Korean J Nephrol. 1991; 10:379–386.
9. Auron A, Simon S, Andrews W, Jones L, Johnson S, Musharaf G, et al. Prevention of peritonitis in children receiving peritoneal dialysis. Pediatr Nephrol. 2007; 22:578–585.

10. Bordador EB, Johnson DW, Henning P, Kennedy SE, McDonald SP, Burke JR, et al. Epidemiology and outcomes of peritonitis in children on peritoneal dialysis in Australasia. Pediatr Nephrol. 2010; 25:1739–1745.

11. Chadha V, Schaefer FS, Warady BA. Dialysis-associated peritonitis in children. Pediatr Nephrol. 2010; 25:425–440.

12. Verrina E, Honda M, Warady BA, Piraino B. Prevention of peritonitis in children on peritoneal dialysis. Perit Dial Int. 2000; 20:625–630.

13. Chiu MC, Tong PC, Lai WM, Lau SC. Peritonitis and exit-site infection in pediatric automated peritoneal dialysis. Perit Dial Int. 2008; 28:Suppl 3. S179–S182.

14. Chua AN, Goldstein SL, Bell D, Brewer ED. Topical mupirocin/sodium hypochlorite reduces peritonitis and exit-site infection rates in children. Clin J Am Soc Nephrol. 2009; 4:1939–1943.

15. Boehm M, Vécsei A, Aufricht C, Mueller T, Csaicsich D, Arbeiter K. Risk factors for peritonitis in pediatric peritoneal dialysis: a single-center study. Pediatr Nephrol. 2005; 20:1478–1483.

16. Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). 2007. Available at: http://spitfire.emmes.com/study/ped/annlrept/annlrept2007.pdf.
17. Rabindranath KS, Adams J, Ali TZ, Daly C, Vale L, Macleod AM. Automated vs continuous ambulatory peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant. 2007; 22:2991–2998.

18. Cnossen TT, Usvyat L, Kotanko P, van der Sande FM, Kooman JP, Carter M, et al. Comparison of outcomes on continuous ambulatory peritoneal dialysis versus automated peritoneal dialysis: results from a USA database. Perit Dial Int. 2011; 31:679–684.

19. Piraino B, Sheth H. Peritonitis-does peritoneal dialysis modality make a difference? Blood Purif. 2010; 29:145–149.

20. Warady BA, Schaefer F, Holloway M, Alexander S, Kandert M, Piraino B, et al. Consensus guidelines for the treatment of peritonitis in pediatric patients receiving peritoneal dialysis. Perit Dial Int. 2000; 20:610–624.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download