Abstract
Purpose
Pneumonia was an important cause of death in 2009 H1N1 influenza pandemic (pH1N1). Clinical characteristics of pH1N1 have been described well, but discriminative characteristics suggesting pH1N1 infection in pneumonia patients are not evident today. We evaluated differences between clinical and radiologic characteristics for those associated and not associated with pH1N1 influenza during the pandemic period.
Materials and Methods
We reviewed all patients with pneumonia who visited the Armed Forces Capital Hospital between July 2009 and February 2010. During this period, all pneumonia patients were tested for pH1N1 by reverse transcription-polymerase chain reaction (RT-PCR) using nasopharyngeal specimens.
Results
In total, 98 patients with pneumonia were enrolled. Their median age was 20 years and all patients were males. Forty-nine (50%) of patients had pH1N1 infection and the others (50%) had negative results in pH1N1 RT-PCR. Patients with pH1N1 infection complained of dyspnea more commonly (83.3% vs. 29.0%; p<0.001), had higher Acute Physiology and Chronic Health Evaluation (APACHE) II scores [5 (range, 0-12) vs. 3 (range, 0-11); p<0.01], fewer days of prehospital illness [2 (range, 0-10) vs. 4 (range, 0-14); p=0.001], and a higher chance of bilateral infiltrates on chest X-ray (CXR) (67.3% vs. 14.3%; p<0.001) and ground-glass opacity (GGO) lesions on computed tomography (CT; 48.9% vs. 22.0%; p<0.001) than patients without pH1N1 infection.
In May 2009, an outbreak of respiratory disease was reported in Mexico, the United States, Canada, and elsewhere. A novel swine-origin influenza A virus (2009 pandemic H1N1 influenza, pH1N1), which contained genes from human, swine, and avian influenza A viruses, was found in the affected patients.1-3 In South Korea, at least 750000 patients were confirmed to have pH1N1 infection between April 2009 and April 2010, with 252 patients dying of the disease.4
The use of antiviral therapy is beneficial in hospitalized patients with pH1N1, especially when initiated at an early stage.5,6 Thus, early diagnosis and treatment is important. The rapid influenza diagnostic test is simple and takes only a few minutes, but the false-negative rate can reach 30-50%.7,8 Reverse transcription-polymerase chain reaction (RT-PCR) remains the definite test for pH1N1, but extensive use is limited due to resources and cost, especially in a pandemic.
Because of such limitations in diagnostic methods, treatment by clinical diagnosis before definitive laboratory results is inevitable in a pandemic. Antiviral drug can be also insufficient. Thus, we should define the clinical characteristics of pH1N1 well for appropriate antiviral therapy and prevention of antiviral agents overuse. To differentiate pH1N1 infection clinically, some nonspecific laboratory abnormalities, such as relative lymphopenia,9 elevated serum transaminases, and an elevated creatinine phosphokinase have been suggested.10 Many studies have been published on the clinical manifestations of pH1N1 pneumonia.5,11-14 However, few information has been reported on direct comparison of clinical and radiological characteristics between pneumonia with and without pH1N1 infection, although pneumonia is the most important complication and cause of death in pH1N1 influenza.15
We evaluated differences between clinical and radiologic characteristics for those associated and not associated with pH1N1 influenza during the pandemic period.
We reviewed all patients who visited the Armed Forces Capital Hospital retrospectively-a central military referral hospital in South Korea-and who were diagnosed with pneumonia between July 2009 and February 2010. In the period of the pH1N1 pandemic, all patients with pneumonia were tested for pH1N1 by RT-PCR using nasopharyngeal specimens, according to the guideline of the Armed Forces Medical Command of Korea. Thus, pH1N1 RT-PCR test results were available for all pneumonia patients at the initial visit. Pneumonia was diagnosed if a new pulmonary infiltrate was associated with at least two of the following factors: a new or increased cough, abnormal temperature (<35.8℃ or >37.8℃), or an abnormal leukocyte count (leukocytosis, leukopenia, or the presence of immature neutrophils). Clinical data on age, gender, body mass index (BMI), and comorbidities, and medical records on symptoms, vital signs, and laboratory results were reviewed. The Acute Physiology and Chronic Health Evaluation (APACHE) II score was calculated to assess the severity of the illness, if data were available. Simple chest radiograph and chest computed tomography (CT) findings were also reviewed by experienced and board-certified radiologists. We also reviewed patients with community-acquired pneumonia who were admitted between July 2008 and February 2009 to exclude the effects of false negative results of pH1N1 RT-PCR, and to confirm that the characteristics of pH1N1 pneumonia were still consistent for this comparison. This study was reviewed and approved by the Institutional Review Board of the Armed Forces Medical Command (AFMC-09-IRB-0014).
Nasopharyngeal specimens were collected from all patients using flock swabs (Flexible Minitip 503CS01 Flock Swabs; Diagnostic Hybrids, Athens, OH, USA). Samples were placed in 3-mL universal transport medium (Diagnostic Hybrids) and stored at 4℃. The specimens were vortexed and nucleic acids were automatically extracted with a NucliSENS easy-MAG instrument (bioMerieux, Einhoven, the Netherlands). Real-time RT-PCR was performed within 12 h of sampling without freezing using RealTime Ready Inf A/H1N1 detection kits (Roche Diagnostics, Basel, Switzerland) and a multiwell plate-based system as described previously. A/H1N1 detection kits contain highly specific primers and probes for detecting influenza A matrix protein 2 and Mexico variant-specific hemagglutinin HA1.
All statistical analyses were performed using SPSS software (ver. 18.0 for Windows; SPSS Inc., Chicago, IL, USA). Categorical variables were compared using Pearson's chi-square test or Fisher's exact test, as appropriate, and continuous variables were compared using Student's t-test or the Mann-Whitney U test. A p value of <0.05 was considered statistically significant.
In total, 98 patients with community-acquired pneumonia were recruited (Fig. 1, Table 1). Their median age was 20 (range, 18-54) years and all patients were males. Forty-nine (50%) patients had laboratory-confirmed pH1N1 infection (group A). The other patients were negative for pH1N1 RT-PCR (group B). No significant differences in BMI or smoking history existed between two groups. No significant difference was seen in the symptoms of cough, sputum, fever, or diarrhea between the two groups, but more patients in group A felt dyspnea than in group B (83.3% vs. 29.0%; p<0.001). Patients in group A had higher APACHE II scores upon presentation than those in group B [5 (range, 0-12) vs. 3 (range, 0-11); p<0.01] and fewer days of prehospital illness [2 (range, 0-10) vs. 4 (range, 0-14); p=0.001]. Among patients with group B, two patients were identified as pneumococcal pneumonia, and three patients were identified as mycoplasma pneumonia.
No significant difference in peak body temperature or systolic and diastolic blood pressure was observed between the groups. Pulse rates were higher in group A than in group B (97.8±21.8 vs. 88.7±12.1; p=0.01). White blood cell and platelet count, C-reactive protein, alanine aminotransferase, creatine kinase, and lactate dehydrogenase levels were similar between the two groups. Alanine aminotransferase levels were slightly lower in group A than group B [19 (range, 10-340) vs. 24 (range, 10-109); p=0.03] (Table 2).
Subjects in group A had more frequent bilateral infiltrates on chest X-ray (CXR) than those in group B (67.3% vs. 14.3%; p<0.001). Similarly, more lobes were involved in the subjects of group A than group B [4 lobes (range, 1-5) vs. 1 lobe (range, 1-5); p<0.001]. Pulmonary infiltrate patterns were significantly different between the groups; ground-glass opacity (GGO) lesions were dominant in group A (22/45, 48.9%) and consolidation was dominant in group B (24/41, 58.5%). Ten (20.4%) patients with pH1N1 influenza had pleural effusions, but this was not significantly different from those without pH1N1 influenza (6/49, 14.6%) (Fig. 2, Table 3).
Fifty-seven patients with community-acquired pneumonia were admitted between July 2008 and February 2009 (group C). More patients in group A felt dyspnea than in group C (83.3% vs. 14.0%; p<0.001). Patients in group A had fewer days of pre-hospital illness [2 (range, 0-10) vs. 6 (range, 1-60); p<0.001] and more frequent bilateral infiltrates on CXR (67.3% vs. 26.3%; p<0.001). More lobes were involved in the subjects of group A than in group C [4 lobes (range, 1-5) vs. 2 lobes (range, 1-5); p<0.001]. GGO lesions were dominant in group A (22/45, 48.9%) and consolidation was dominant in group C (32/54, 60.4%) (Table 4).
Antiviral therapy (oseltamivir) was administered to all patients in group A, and 36 of 49 (73.5%) patients in group B also took antiviral therapy. Days from symptom onset to oseltamivir administration was significantly shorter in group A than in group B [2 (range, 0-5) vs. 4 (range, 0-14); p=0.001]. No significant difference between days to afebrile and days of hospitalization was observed between the groups. One patient in group A needed both mechanical ventilation and a vasopressor during hospitalization. This patient showed bilateral pneumonic consolidations and pleural effusions on chest radiograph and CT and impaired left ventricular function on echocardiography. After 9 days of antiviral and antibiotics therapy, this patient was weaned from mechanical ventilation. No patient required mechanical ventilation in group B, but two patients needed vasopressors.
We analyzed how accurately clinical and radiographic parameters could differentiate pneumonia with pH1N1 influenza from those without pH1N1. If patients with pneumonia had dyspnea, they had a pH1N1 infection with a positive predictive value (PPV) of 0.82 [95% confidence interval (CI) 0.63-0.92] and the positive likelihood ratio (LR+) was 2.87 (95% CI 1.62-5.09). If these patients also had bilateral infiltration on CXR, we could say they had pH1N1 infection with a PPV of 0.88 (95% CI 0.70-0.96), although the negative predictive value (NPV) fell to 0.77 (95% CI 0.59-0.88). After adding GGO on CT to dyspnea and bilateral infiltration on CXR, the PPV and specificity were almost the same, despite decreased NPV and sensitivity (Table 5).
We directly compared the clinical and radiographic characteristics of pneumonia with and without pH1N1 influenza because all patients with pneumonia had RT-PCR results for pH1N1 in the pandemic period. This patient cohort showed that patients with pH1N1 influenza had a higher probability of dyspnea, and they visited the hospital earlier than patients without pH1N1 influenza. More than two-thirds of them showed bilateral infiltration and GGO on chest radiographs, which were different from those patients without pH1N1 influenza, whose dominant radiographic findings were mono-lobar involvement and consolidation.
The clinical effects of pH1N1 vary from subclinical illness to severe respiratory failure and death, and resemble those observed in patients with seasonal influenza.5,16,17 However, some clinical characteristics of pH1N1 differ from those of seasonal influenza, such as younger age and less comorbidity.18 In our study, dyspnea and fever were more prevalent in patients with pH1N1 influenza-associated pneumonia. Current guidelines recommend that dyspnea should be considered a warning sign for severer disease.19
Previous reports demonstrated that multifocal GGO was the dominant lesion in pH1N1-associated pneumonia.9,20-22 Our study showed that these predominant radiographic findings were significantly different from those without pH1N1 influenza. Multifocal GGOs are attributable to partial filling of air spaces, thickening of interstitial tissues, or increased capillary blood volume.23,24 Atypical infection, such as Mycoplasma, Chlamydia, or Legionella infection and eosinophilic pneumonia can be suggested in the differential diagnosis for this radiographic finding.25,26 In our study, multi-lobar and bilateral involvement was also a characteristic feature of pH1N1 pneumonia, which was different from seasonal influenza in a previous study.18 Ten (20.4%) patients with pH1N1 influenza had pleural effusions, but this was not significantly different from those without pH1N1 influenza. No subject was diagnosed with a pulmonary embolism, which was reported as a complication of influenza;9,27,28 centrilobular nodules and tree-in-bud opacities were not noted.
We evaluated whether clinical and radiographic parameters could predict pH1N1 association in the usual diagnostic process for pneumonia. We used dyspnea, bilateral infiltration on CXR, and GGO on CT as predictive markers for pH1N1, which were more prevalent among pH1N1-associated pneumonia. In this study population, we could say that patients with dyspnea had the possibility of pH1N1 infection, with a PPV of 0.74. The PPV increased to 0.88 if bilateral infiltration was also noted on CXR. These data warrant prompt antiviral therapy for patients who have pneumonia with dyspnea and bilateral infiltration on CXR in a pandemic, especially when a confirmative diagnostic test is not immediately available. GGO on CT did not further increase diagnostic yield. This may be explained by some patients having atypical pneumonia, but not pH1N1, and having characteristics similar to those of pH1N1 pneumonia.
In this cohort, no patient died. In fact, no mortality was reported for pH1N1 influenza in the Korean military during the 2009 pandemic. This may be a peculiar finding, considering the mortality of pH1N1-associated pneumonia was 7.1% (19/269) during the same period in Korea29 and pneumonia has been an important contributor to pH1N1 mortality.15 Several factors may explain this. First, our cohort consisted of young adults. Previous reports in Korea showed that the fatal cases were typically older than the survivors and most of the deceased were over 50 years old.29 Second, soldiers were vaccinated 2 months earlier than the general population, according to the vaccination priority policy in Korea. Third, prophylactic and therapeutic oseltamivir could be supplied earlier than to the general population during the pandemic. Considering the communal setting of military life, military doctors tried to find patients by active surveillance and tried to prescribe medication as early as possible to prevent outbreaks. Several observational studies have shown that early treatment with oseltamivir may reduce the risk of progression to severe disease, requiring intensive care unit admission or resulting in death.5,30,31
This study has several limitations. First, this was a single-center study and generalizing the results may not be possible. However, this study was performed in a military hospital in Southern Gyeonggi Province, and soldiers in this area were referred to this hospital through the military medical system. Thus, the study population represented young adults with pneumonia in this area. Second, it was a retrospective study, based on a review of medical records. Although all patients with pneumonia had information about their pH1N1 influenza status, some descriptions about symptoms were missing and we could not compare symptoms for all patients. Hospital stay was also hard to compare because the patients stayed in hospital for relatively long period for isolation from their military units. Third, only a small number of patients had the probable pathogen isolated. Although previous studies also reported only a small portion of patients with pneumonia as having the pathogen isolated,32 the proportion in this study is still lower. In addition, atypical organisms might have been a pathogen in some patients. However, some patients should have false negative results in RT-PCR of H1N1 influenza, which was examined only at their initial visit. The viral load of influenza could be lower at the initial visit and we did not repeat RT-PCR tests. Previous anti-viral medication could also affect the RT-PCR results, although anti-viral therapy such as oseltamivir was not widely available in Korea military unit. To overcome this limitation, we compared the characteristics of patients with pneumonia during the same season of previous year and it showed consistent results. Although some patients with negative RT-PCR of H1N1 were included in group A, it did not affect the main results.
In conclusion, dyspnea, GGO on CT, and multi-lobar involvement were more common in pH1N1 influenza-associated pneumonia. Our study will help prompt antiviral therapy in a more efficient way, if another pandemic is encountered, especially when rapid laboratory confirmation is not available and anti-viral agents are insufficient.
Figures and Tables
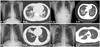 | Fig. 2Typical radiographic findings of pneumonia with and without pH1N1 influenza infection. (A) CXR (a) and CT (b) findings of 21-year-old (A-1) and 23-year-old man (A-2) with pH1N1 influenza infection. CXR and CT showed bilateral infiltrates and GGO-dominant infiltrations in multiple lobes. (B) CXR (a) and CT (b) findings of the 21-year-old (B-1) and 20-years-old man (B-2) without pH1N1 influenza infection. CXR and CT showed unilateral consolidation and unilobar consolidation. CXR, chest X-ray; CT, computed tomography; GGO, ground-glass opacity. |
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D project, Ministry for Health, Welfare & Family Affairs, and Republic of Korea (A120301).
References
1. Centers for Disease Control and Prevention (CDC). Update: infections with a swine-origin influenza A (H1N1) virus--United States and other countries, April 28, 2009. MMWR Morb Mortal Wkly Rep. 2009; 58:431–433.
2. Centers for Disease Control and Prevention (CDC). Swine influenza A (H1N1) infection in two children--Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009; 58:400–402.
3. Centers for Disease Control and Prevention (CDC). Update: swine influenza A (H1N1) infections--California and Texas, April 2009. MMWR Morb Mortal Wkly Rep. 2009; 58:435–437.
4. Kim WJ. Pandemic influenza (H1N1 2009): experience and lessons. Infect Chemother. 2010; 42:61–63.

5. Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009; 361:1935–1944.

6. Viasus D, Paño-Pardo JR, Pachón J, Riera M, López-Medrano F, Payeras A, et al. Timing of oseltamivir administration and outcomes in hospitalized adults with pandemic 2009 influenza A(H1N1) virus infection. Chest. 2011; 140:1025–1032.

7. Cunha BA. Swine Influenza (H1N1) pneumonia: clinical considerations. Infect Dis Clin North Am. 2010; 24:203–228.

8. Riquelme R, Riquelme M, Rioseco ML, Inzunza C, Gomez Y, Contreras C, et al. Characteristics of hospitalised patients with 2009 H1N1 influenza in Chile. Eur Respir J. 2010; 36:864–869.

9. Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009; 193:1488–1493.

10. Cunha BA, Pherez FM, Strollo S. Swine influenza (H1N1): diagnostic dilemmas early in the pandemic. Scand J Infect Dis. 2009; 41:900–902.

11. Rello J, Rodríguez A, Ibañez P, Socias L, Cebrian J, Marques A, et al. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009; 13:R148.

12. Denholm JT, Gordon CL, Johnson PD, Hewagama SS, Stuart RL, Aboltins C, et al. Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med J Aust. 2010; 192:84–86.
13. Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009; 302:1896–1902.

14. Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009; 302:1872–1879.

15. Soto-Abraham MV, Soriano-Rosas J, Díaz-Quiñónez A, Silva-Pereyra J, Vazquez-Hernandez P, Torres-López O, et al. Pathological changes associated with the 2009 H1N1 virus. N Engl J Med. 2009; 361:2001–2003.

16. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009; 360:2605–2615.

17. Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009; 361:674–679.

18. Riquelme R, Torres A, Rioseco ML, Ewig S, Cillóniz C, Riquelme M, et al. Influenza pneumonia: a comparison between seasonal influenza virus and the H1N1 pandemic. Eur Respir J. 2011; 38:106–111.

19. World Health Organization. WHO guidelines for pharmacological management of pandemic (H1N1) 2009 influenza and other influenza viruses. 2010. accessed on 2011 July 15. Geneva: Available at: http://www.who.int/csr/resources/publications/swineflu/h1n1_use_antivirals_20090820/en/index.html.
20. Mori T, Morii M, Terada K, Wada Y, Kuroiwa Y, Hotsubo T, et al. Clinical characteristics and computed tomography findings in children with 2009 pandemic influenza A (H1N1) viral pneumonia. Scand J Infect Dis. 2011; 43:47–54.

21. Ajlan AM, Quiney B, Nicolaou S, Müller NL. Swine-origin influenza A (H1N1) viral infection: radiographic and CT findings. AJR Am J Roentgenol. 2009; 193:1494–1499.

22. Mollura DJ, Asnis DS, Crupi RS, Conetta R, Feigin DS, Bray M, et al. Imaging findings in a fatal case of pandemic swine-origin influenza A (H1N1). AJR Am J Roentgenol. 2009; 193:1500–1503.

23. Remy-Jardin M, Remy J, Giraud F, Wattinne L, Gosselin B. Computed tomography assessment of ground-glass opacity: semiology and significance. J Thorac Imaging. 1993; 8:249–264.
24. Remy-Jardin M, Giraud F, Remy J, Copin MC, Gosselin B, Duhamel A. Importance of ground-glass attenuation in chronic diffuse infiltrative lung disease: pathologic-CT correlation. Radiology. 1993; 189:693–698.

25. John SD, Ramanathan J, Swischuk LE. Spectrum of clinical and radiographic findings in pediatric mycoplasma pneumonia. Radiographics. 2001; 21:121–131.

26. King MA, Pope-Harman AL, Allen JN, Christoforidis GA, Christoforidis AJ. Acute eosinophilic pneumonia: radiologic and clinical features. Radiology. 1997; 203:715–719.

27. Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009; 361:680–689.

28. Ohrui T, Takahashi H, Ebihara S, Matsui T, Nakayama K, Sasaki H. Influenza A virus infection and pulmonary microthromboembolism. Tohoku J Exp Med. 2000; 192:81–86.

29. Choi WI, Yim JJ, Park J, Kim SC, Na MJ, Lee WY, et al. Clinical characteristics and outcomes of H1N1-associated pneumonia among adults in South Korea. Int J Tuberc Lung Dis. 2011; 15:270–275. i
30. Louie JK, Acosta M, Jamieson DJ, Honein MA. California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010; 362:27–35.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download