Abstract
Purpose
To investigate whether preoperative serum anti-mullerian hormone (AMH) levels are lower in women with ovarian endometrioma and in women with mature cystic teratoma of the ovaries.
Materials and Methods
In a tertiary university hospital, a retrospective case-control study was performed. Serum AMH levels between an advanced (stage III and IV) endometrioma group (n=102) and an age- and body mass index (BMI)-matched control group were compared. Serum AMH levels between an ovarian mature cystic teratoma group (n=48) and age- and BMI-matched controls were also compared.
Results
Absolute serum AMH and multiples of the median for AMH (AMH-MoM) relevant to Korean standards were lower in the endometrioma group than controls, but this was not statistically significant (mean±SEM, 2.9±0.3 ng/mL vs. 3.3±0.3 ng/mL, p=0.28 and 1.3±0.1 vs. 1.6±0.1, p=0.29, respectively). Specifically, the stage IV endometriosis group (n=51) exhibited significantly lower serum AMH and AMH-MoM (2.1±0.3 vs. 3.1±0.4 ng/mL, p=0.02 and 1.1±0.1 vs. 1.7±0.2, p=0.03, respectively). Serum AMH and AMH-MoM levels were similar between stage III endometriosis and controls (3.7±0.5 vs. 3.4±0.5 ng/mL and 1.6±0.2 vs. 1.5±0.2, respectively), as well as between the mature cystic teratoma group and controls (4.0±0.5 ng/mL vs. 4.0±0.5 ng/mL and 1.6±0.2 vs. 1.6±0.3, respectively). Interestingly, AMH-MoM level was negatively correlated with endometriosis score with statistical significance (r2=0.13, p<0.01).
Conclusion
In women with advanced ovarian endometrioma, preoperative serum AMH values tended to be lower than those for age and BMI-matched controls. Notably, stage IV endometrioma appeared to be closely associated with decreased ovarian reserve, even before operation. Clinicians should keep this information in mind before undertaking surgery of ovarian endometrioma.
Endometriosis is a frequent gynecologic disease affecting 5% of women of reproductive age and up to 40% in infertile patients.1 It has been reported that the likelihood of having this disease is 6-8 times higher in infertile women than in those that are fertile.2 Several studies have advocated for a relationship between endometriosis and infertility, and it is now generally accepted that endometriosis lowers fecundity. Although the mechanism is still unclear, pelvic adhesion, ovulatory dysfunction, decreased oocyte quality and impaired implantation have been proposed as mechanisms of decreased fecundity in endometriosis patients.3,4 A previous study on pregnancy rate in in vitro fertilization (IVF) reported relatively poor outcomes in endometriosis-associated infertility.5 However, a subsequent report showed similar outcomes between endometriosis-associated infertility and tubal factor infertility.6
Recently, Kitajima, et al.7 evaluated the histologic features of ovarian cortical tissue in endometriomas and found significantly lower follicular density than that for contralateral normal ovaries; they also found a presence of fibrosis and a loss of cortex-specific stroma in tissues from endometriomas. They demonstrated an association between tissue alteration in endometriomas and reduced ovarian reserve. This finding indicated that endometriosis itself could reduce ovarian reserves. Furthermore, several studies have shown a significant difference in ovarian reserve according to endometriosis severity.8
Previous study noted that infertile patients with endometriosis exhibit decreased serum anti-mullerian hormone (AMH) levels compared to male factor infertility, and advocated that measuring AMH may be beneficial in optimizing the dose needed for gonadotropin treatment prior to ovarian stimulation.8 Moreover, further research suggested similarities in ovarian reserve in infertility patients with minimal/mild endometriosis compared to patients with tubal obstruction.9 Although a few studies have set out to compare serum AMH level in women with endometrioma and controls before surgery, they have yet to find significant reductions in serum AMH levels in women with endometrioma prior to surgery.10-12
Mature cystic teratomas are the most common ovarian tumors, occupying up to 20% of all ovarian tumors.13 About 70% of patients are diagnosed between the ages of 20 to 49 years old, i.e., reproductive age.14 Despite its high prevalence, studies have frequently focused on malignant transformation, because it is the most commonly encountered germ cell neoplasm of the ovaries. Although one previous study showed an increased prevalence of infertility in women with mature cystic teratomas of ovary,15 direct evidence of an association thereof is lacking.
The present study aimed to verify the hypothesis that ovarian reserves are reduced in patients with ovarian endometriosis or mature cystic teratomas. To do so, we compared preoperative serum AMH values between women with ovarian endometriomas or teratomas and age- and body mass index (BMI)-matched controls without the disease.
We reviewed the medical records of 997 patients in whom serum AMH level was measured between October 2009 and July 2011 at the Seoul National University Bundang Hospital. The Institutional Review Board of Seoul National University Bundang Hospital approved the use patient medical records to conduct this study (B-1201/143-114).
In total, 102 patients in whom advanced endometriosis (n=51 for stage III, n=51 for stage IV) was confirmed by postoperative pathologic examination were selected. All selected patients exhibited ovarian endometrioma unilaterally (n=67) or bilaterally (n=35). The size and bilaterality of ovarian endometrioma as well as its stage and score as evaluated by the revised American Society of Reproductive Medicine scoring system were identified in the operation record. We selected a control group by age (±1 year) and BMI (±10%) matching from each patient of the study group. None of the subjects in the control group had ovarian disease including polycystic ovary syndrome, which was confirmed surgically (n=67) or ultrasonographically (n=35); sixty-seven control patients without ovarian disease received operation due to uterine leiomyomas and 35 controls underwent ultrasonography for gynecological screening. None of the controls had a history of ovarian surgery. Gynecologic history such as menstrual cycle irregularity, parity, infertility, and other systemic diseases were also reviewed. We excluded the subjects who had history of oral contraceptive, ovulation induction agents or GnRH agonist use.
For mature cystic teratomas of the ovaries, 48 patients and 48 control subjects without ovarian disease were selected. In the control group, the absence of ovarian disease was confirmed surgically (n=24) or ultrasonographically (n=24). The method for selecting controls was the same as described above.
Serum AMH level was measured by a commercial kit (Beckman Coulter Immunotech, Marseille, France). Chi-square test was used for the comparison of proportions between both groups. Paired Student's t-test was used to compare the parameters between two groups if the data showed a normal distribution. If not, Wilcoxon signed rank test was used. Serum AMH values were compared as its absolute value as well as multiples of the median (MoM), which was calculated by using the age-specific reference value for AMH in Koreans.16 Multivariate analysis was also performed for identifying factors affecting serum AMH level. All p-values <0.05 were considered statistically significant. Data were shown as mean±standard error of mean.
There were no significant differences in age, BMI and gynecological parameters such as cycle irregularity, parity, infertility, and systemic diseases between subjects in the ovarian endometrioma group and controls (Table 1). Preoperative serum AMH level in the endometriosis group was not significantly different compared to controls. However, when analyzed by endometriosis stage separately, AMH level was significantly lower in stage IV endometriosis subjects compared to controls. AMH-MoM value was also significantly lower in the stage IV endometriosis group only.
Preoperative serum AMH levels were 3.1±0.4 ng/mL in the unilateral endometriosis group and 2.5±0.4 ng/mL in the bilateral endometrioma group (p=0.34). AMH-MoM value was not different according to bilaterality (1.4±0.1 vs. 1.1±0.2, p=0.10). Multivariate analysis revealed that preoperative serum AMH levels and AMH-MoM values were not affected by the bilaterality of the endometrioma. In the study group, the mean size of endometrioma was 5.5±0.2 cm in diameter, and multivariate analysis revealed that cyst size was not correlated with serum AMH level or AMH-MoM value. Interestingly, AMH-MoM level was negatively correlated with endometriosis score with statistical significance (r2=0.13, p<0.01) (Fig. 1).
Between the mature cystic teratoma group and controls, there were no significant differences in age, BMI, clinical parameters and preoperative serum AMH level or AMH-MoM value (Table 2). The bilaterality of teratoma was also not shown to be relevant to serum AMH level (unilateral 3.0±0.3 vs. bilateral 4.0±0.6, p=0.62) or AMH-MoM value. The mean size of teratoma was 6.3±0.3 cm in diameter, and showed no correlation with serum AMH level or AMH-MoM value. Multivariate analysis revealed that preoperative serum AMH level and AMH-MoM value was not affected by any clinical parameter.
AMH as a dimeric glycoprotein is secreted by preantral and early antral follicles. Production of AMH begins during the fetal period and the highest level of AMH is achieved at puberty. Afterwards, AMH levels decline with aging till it reaches nearly zero at menopause.17 Moreover, AMH level is associated with ovarian follicle pool size,18 which decreases proportionally with aging. Accordingly, AMH was shown to be a better marker for ovarian reserve than FSH or inhibin B in a previous study.19 To date, AMH has been commonly measured to evaluate ovarian reserves in infertile women or women who want to know their ovarian reserves. It can also be measured in women planning to undergo ovarian surgery, especially in young women, because ovarian reserves could be decreased after conservative operation.10 AMH level is quite stable throughout the menstrual cycle and does not show circadian variation.20,21 Interpretation of AMH value should include the subject's BMI because AMH level tends to be lower in obese women.22 Hence, it is reasonable that study and control groups should be selected by age- and BMI-matching in any comparative study.
Although endometriosis is generally considered to lower fertility, its exact mechanism on fecundity is not clear.23 Several studies supported poorer IVF outcomes in endometriosis patients.4,24,25 Nevertheless, contradictory results also exist.26,27 The majority of previous studies regarding AMH value in endometriosis patients have focused on infertile patients participating in IVF.8,28 However, to explain the risk of reduced fecundity in endometriosis patients, ovarian reserve should be evaluated among the general population, regardless of existence of infertility.
We observed that preoperative serum AMH level and/or AMH-MoM level was significantly lower in women with stage IV endometriosis than women without endometriosis, but this difference was not observed in women with stage III endometriosis. From our observations, severe endometriosis may affect ovarian reserves. Accordingly, we propose that clinicians should measure serum AMH levels prior to surgery of ovarian endometriomas. If advanced stage is suspected, measurement of preoperative AMH levels would be more important, because they could decline even after conservative ovarian surgery. Considering that endometriosis is a progressive disease, our findings are biologically plausible because ovarian reserves before surgery significantly decrease in women with stage IV endometriosis but not in stage III. Furthermore, we found for the first time that AMH-MoM level is negatively correlated with endometriosis score. Among our result, the corresponding endometriosis score was -56.5 for 1.0 MoM; this means that preoperative serum AMH level tended to be lower than the median for women of the same age group with scores >56.5. When the cut-off value for endometriosis score was set as 56.5, the percent of women with serum AMH level under 1.0 MoM was 52.6% (40/76) in women with a score below 56.5 and 53.8% (14/26) in women with a score above 56.5 (p>0.05). In the present study, we excluded patients with stage I and II endometriosis because the number of subjects was relatively small. In fact, it is not easy to select adequate number of control women in cases of early stage endometriosis.
The causes of decreased ovarian reserve in women with endometriosis are not clear. A previous study suggested a relationship with inflammation.29 Others have suggested that TNF in follicular fluids affects oocyte quality.30 In the operation field, ovarian follicles are firmly adjacent to endometriosis lesions, which is not common in other ovarian cysts such as teratomas. We speculate that endometriosis lesions may invade the ovarian cortex and cause damage to ovarian follicles directly.
Mature cystic teratoma is the most common ovarian tumor in women of reproductive age.13 Despite its high prevalence, studies about its effect on ovarian reserves are scarce. In the present study, no difference in serum AMH level was observed between women with mature cystic teratomas and age- and BMI-matched controls. We speculate that mature cystic teratomas cause simple mechanical tissue stretching and do significantly not affect ovarian reserves.
To the best of our knowledge, this is the first report regarding decreased AMH level in women with advanced endometriosis. Ovarian reserves in patients with advanced endometriosis tended to be lower than those in age-matched controls even before surgery; thus, clinicians should keep this information in mind before surgery for ovarian endometrioma. Further large-scaled, prospective study would be necessary to confirm our findings. Additionally, further exploration of the exact mechanisms of lowered ovarian reserve in advanced endometriosis is warranted.
Figures and Tables
 | Fig. 1Preoperative serum AMH level (presented as multiples of the median calculated by using the age-specific reference value for AMH in Koreans) and postoperative endometriosis rASRM score. The solid line represents a regression line and two dashed lines represent ±95% confidence lines. AMH, anti-mullerian hormone; rASRM, revised American Society of Reproductive Medicine; EMS, endometriosis. |
Table 1
Gynecological Parameters and Preoperative Serum AMH Values between Women with Endometrioma and Age and Body Mass Index-Matched Controls
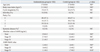
AMH, anti-mullerian hormone; MoM, multiples of the median; NS, not significant.
AMH-MoM was calculated by using the age-specific reference value for AMH in Koreans.16
*Paired Student t-test.
†Chi-square test.
‡Wilcoxon signed rank test.
§Thyroid cancer, Behcet's disease, Chronic hepatitis, or breast cancer.
Table 2
Gynecological Parameters and Preoperative Serum AMH Values between Women with Teratoma and Age and Body Mass Index-Matched Controls
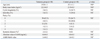
AMH, anti-mullerian hormone; MoM, multiples of the median; NS, not significant.
AMH-MoM was calculated by using the age-specific reference value for AMH in Koreans.16
*Paired Student t-test.
†Chi-square test.
‡Wilcoxon signed rank test.
§Multiple myeloma, Pulmonary Tuberculosis.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A120043).
References
1. Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci. 2008; 1127:92–100.
2. Verkauf BS. Incidence, symptoms, and signs of endometriosis in fertile and infertile women. J Fla Med Assoc. 1987; 74:671–675.
3. Navarro J, Garrido N, Remohí J, Pellicer A. How does endometriosis affect infertility? Obstet Gynecol Clin North Am. 2003; 30:181–192.

4. Lessey BA. Implantation defects in infertile women with endometriosis. Ann N Y Acad Sci. 2002; 955:265–280.

5. Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002; 77:1148–1155.

6. Wright VC, Chang J, Jeng G, Macaluso M. Centers for Disease Control and Prevention (CDC). Assisted reproductive technology surveillance--United States, 2005. MMWR Surveill Summ. 2008; 57:1–23.
7. Kitajima M, Defrère S, Dolmans MM, Colette S, Squifflet J, Van Langendonckt A, et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil Steril. 2011; 96:685–691.

8. Shebl O, Ebner T, Sommergruber M, Sir A, Tews G. Anti muellerian hormone serum levels in women with endometriosis: a case-control study. Gynecol Endocrinol. 2009; 25:713–716.

9. Campos CS, Vaamonde D, Andreoli C, Martins AC, Genro VK, Souza CA, et al. Follicular-fluid anti-Müllerian hormone concentration is similar in patients with endometriosis compared with non-endometriotic patients. Reprod Biomed Online. 2010; 21:470–473.

10. Chang HJ, Han SH, Lee JR, Jee BC, Lee BI, Suh CS, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010; 94:343–349.

11. Iwase A, Hirokawa W, Goto M, Takikawa S, Nagatomo Y, Nakahara T, et al. Serum anti-Müllerian hormone level is a useful marker for evaluating the impact of laparoscopic cystectomy on ovarian reserve. Fertil Steril. 2010; 94:2846–2849.

12. Kitajima M, Khan KN, Hiraki K, Inoue T, Fujishita A, Masuzaki H. Changes in serum anti-Müllerian hormone levels may predict damage to residual normal ovarian tissue after laparoscopic surgery for women with ovarian endometrioma. Fertil Steril. 2011; 95:2589–2591.e1.

13. Kim MJ, Kim NY, Lee DY, Yoon BK, Choi D. Clinical characteristics of ovarian teratoma: age-focused retrospective analysis of 580 cases. Am J Obstet Gynecol. 2011; 205:32.e1–32.e4.

14. Lakkis WG, Martin MC, Gelfand MM. Benign cystic teratoma of the ovary: a 6-year review. Can J Surg. 1985; 28:444–446.
15. Parazzini F, La Vecchia C, Negri E, Moroni S, Villa A. Risk factors for benign ovarian teratomas. Br J Cancer. 1995; 71:644–646.

16. Lee JY, Jee BC, Lee JR, Kim CH, Park T, Yeon BR, et al. Age-related distributions of anti-Müllerian hormone level and anti-Müllerian hormone models. Acta Obstet Gynecol Scand. 2012; 91:970–975.

17. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002; 77:357–362.

18. Kunt C, Ozaksit G, Keskin Kurt R, Cakir Gungor AN, Kanat-Pektas M, Kilic S, et al. Anti-Mullerian hormone is a better marker than inhibin B, follicle stimulating hormone, estradiol or antral follicle count in predicting the outcome of in vitro fertilization. Arch Gynecol Obstet. 2011; 283:1415–1421.

19. Muttukrishna S, McGarrigle H, Wakim R, Khadum I, Ranieri DM, Serhal P. Antral follicle count, anti-mullerian hormone and inhibin B: predictors of ovarian response in assisted reproductive technology? BJOG. 2005; 112:1384–1390.

20. Bungum L, Jacobsson AK, Rosén F, Becker C, Yding Andersen C, Güner N, et al. Circadian variation in concentration of anti-Müllerian hormone in regularly menstruating females: relation to age, gonadotrophin and sex steroid levels. Hum Reprod. 2011; 26:678–684.

21. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006; 21:3103–3107.

22. Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF 3rd. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007; 87:101–106.

23. Osuga Y, Koga K, Tsutsumi O, Yano T, Maruyama M, Kugu K, et al. Role of laparoscopy in the treatment of endometriosis-associated infertility. Gynecol Obstet Invest. 2002; 53:Suppl 1. 33–39.

24. Arici A, Oral E, Bukulmez O, Duleba A, Olive DL, Jones EE. The effect of endometriosis on implantation: results from the Yale University in vitro fertilization and embryo transfer program. Fertil Steril. 1996; 65:603–607.

25. Azem F, Lessing JB, Geva E, Shahar A, Lerner-Geva L, Yovel I, et al. Patients with stages III and IV endometriosis have a poorer outcome of in vitro fertilization-embryo transfer than patients with tubal infertility. Fertil Steril. 1999; 72:1107–1109.

26. Al-Azemi M, Bernal AL, Steele J, Gramsbergen I, Barlow D, Kennedy S. Ovarian response to repeated controlled stimulation in in-vitro fertilization cycles in patients with ovarian endometriosis. Hum Reprod. 2000; 15:72–75.

27. Díaz I, Navarro J, Blasco L, Simón C, Pellicer A, Remohí J. Impact of stage III-IV endometriosis on recipients of sibling oocytes: matched case-control study. Fertil Steril. 2000; 74:31–34.

28. Falconer H, Sundqvist J, Gemzell-Danielsson K, von Schoultz B, D'Hooghe TM, Fried G. IVF outcome in women with endometriosis in relation to tumour necrosis factor and anti-Müllerian hormone. Reprod Biomed Online. 2009; 18:582–588.

29. Halis G, Arici A. Endometriosis and inflammation in infertility. Ann N Y Acad Sci. 2004; 1034:300–315.

30. Lee KS, Joo BS, Na YJ, Yoon MS, Choi OH, Kim WW. Relationships between concentrations of tumor necrosis factor-alpha and nitric oxide in follicular fluid and oocyte quality. J Assist Reprod Genet. 2000; 17:222–228.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download