Abstract
Purpose
To evaluate the concordance of cancer location of the tissue mapping from a mechanical pressure transducer with magnetic resonance imaging (MRI) scans.
Materials and Methods
A total of 60 indentations were performed on 5 prostate specimens obtained after radical prostatectomy utilizing a robotic indentation system. The mechanical elastic moduli of suspected malignant lesions were calculated and mapped, and their locations were compared with suspicious areas of malignancy on MRI scans.
Results
The concordance rate between the location mapping from the robotic indentation system and MRI scans results was 90.0% (54/60). The sensitivity and specificity of the robotic indentation system were 87.9% (29/33) and 92.6% (25/27), respectively. The positive predictive value and negative predictive value were 93.5% (29/31) and 93.1% (27/29), respectively.
Conclusion
The locations of malignant lesions derived from our robotic indentation system correlated strongly with the locations of suspected areas of malignancy on MRI scans. Our robotic system may provide a more targeted biopsy of the prostate than conventional non-targeted systemic biopsy, possibly improving the diagnostic accuracy of prostatic biopsies for cancer.
Prostate cancer is the second-leading cause of cancer death in men after lung cancer, with an estimated 33720 related deaths reported in 2011.1 Prostate cancer accounted for 240890 (29%) of all newly diagnosed cases of cancer, making it the commonest internal malignancy in the United States in 2011.1 Screening for prostate cancer had resulted in a stage migration of the disease, allowing cancers to be diagnosed and treated earlier with improved cancer specific survival. Indeed, mortality rates from prostate cancer continued to fall over the years since 1990.1 Screening for prostate cancer currently involves serum prostate specific antigen (PSA) and digital rectal examination (DRE). However, the sensitivities and specificities of these screening tools, either alone or in combination, are low.
The optimal method of screening for prostate cancer has yet to be established. DRE is neither objective nor quantitative in screening for cancer. Physicians perform DRE by inserting a lubricated, gloved finger into the rectum of patients, feeling for areas suspicious for malignancy. Although DRE can be easily performed at consultation and has almost no risks, the findings are highly subjective and dependent of the physician's experience and expertise. Abnormal DRE had been shown to be an independent predictor of high-grade2 and clinically significant tumors.3,4 However, various authors have reported dismal positive predictive value (PPV) of DRE, ranging from 28.0%5 to 33%.6
Technological advancement had led to mechanical systems providing tactile and kinesthetic feedback to the operator.7 Ahn, et al.8 reported differences in the mean elastic modulus of regions containing cancer and normal prostate tissues, using a motorized indenter in ex vivo experiments. They showed that the elastic modulus was quantitatively greater in tissues with a Gleason score of 8 or with a tumor volume >5 cm3 when compared with normal tissues.
It has been shown that the suspicious areas on magnetic resonance imaging (MRI) scans correlate well with location of malignant lesions in post-radical prostatectomy pathological specimens.7,9 In our present study, we aim to evaluate the correlation of the tissue mapping from our robotic indentation system with suspicious areas suggestive of malignancy on MRI scans.
All patients provided written informed consent, and the study was approved by the Institutional Review Board of Severance Hospital (IRB No: 1-2011-0048). Prostate specimens were obtained from 5 patients who had undergone radical prostatectomy at Severance Hospital, Yonsei University in Seoul, Korea, between October 2011 and March 2012. Patients who had received preoperative hormonal/radiation therapy or prostate-related surgery were excluded from the study. Patients with clinically insignificant small cancer (tumour volume <0.5 mL) were also excluded from the study. We used C-500 sensor (Tactarray, Los Angels, CA, USA) as our robotic palpation module.
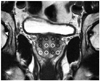
In order to simulate rectal examination, a 3-dimensional model comprising of morphology of the bones and ligaments around the prostate was constructed. An artificial bony pelvis was also created with a 3-dimensional printer (rapid prototype) and the prostatic specimen was placed into its 'anatomical' position (Fig. 1). A DRE simulator model (MK-2, Limbs and things, Savannah, GA, USA) and two rotary encoders (E20s, Autonics, Bucheon, Gyeonggi, Korea) were utilized to measure the angle of yaw and pitch direction. The investigator who carried out the experiments was blinded to all clinical information.
We used our robotic system to perform the palpation examination for localization of abnormal regions in 5 prostate glands. This was done in 5 sequential steps. Firstly, the tip of the probe was positioned in contact with the posterior surface of the prostate. Secondly, the prostatic tissue was indented with another 2 mm diameter probe at a rate of 1 mm/second to produce a 3 mm deep deformation in the tissue. Thirdly, when the deformation was induced, a reaction force occurred. This reaction force from the indented tissue was then measured by a force sensor in the fourth step. Finally, the reaction force and deformation data were acquired via a data acquisition system. All experiments were performed in the operating room within 30 minutes of extraction of the specimen. For each prostate specimen, the indentations were performed at a pre-determined 12 sites across the posterior surface of the prostate as shown in Fig. 2. These 12 sites were similar to those of double-sextant needle biopsies, including the lateral apex, lateral-mid, lateral base, medial apex, medial mid, and medial base. A total of 60 indentations were performed on the 5 specimens.
The finite element method (FEM) simulation was performed using the commercial software ABAQUS/Standard 6.5.1 (SIMULIA, Providence, RI, USA). The estimated elastic moduli were compared with the local normal criteria in order to diagnose and localize prostate cancer.8 The local reaction force was measured and the tissue's local elastic modulus was then estimated using the prostate model. Local normal criteria referred to the range between the upper bound (average+standard deviation) and the lower bound (average value-standard deviation) values. Localisation of any prostatic lesion was performed by comparing the estimated local elastic modulus and the normal tissue criteria, and a tissue mapping was obtained. We then compared the tissue mapping with suspicious areas of malignancies on MRI scans. A clinical radiologist, blinded to all relevant information, reported on suspicious malignant lesions on the MRI according to the 12 areas measured by the robotic system, as described earlier.
A total of 5 prostate specimens were analyzed. The mean age of the patients was 59.4±3.5 years (range 51-70 years), and the mean PSA level was 11.9±8.6 ng/mL (range, 4.9-22.6 ng/mL). The mean preoperative volume of the prostate was 26.3±8.3 mL (range, 18.3-39.4 mL). The pathologic tumor stage was T2c in 1 patient, T3a in 2 patients, and T3b in 2 patients.
Ex vivo experiments were performed for the 5 resected human prostate specimens. Overall, the mechanical properties of 60 regions from 5 prostate specimens were calculated from the FEM-based mechanical property characterization. As shown in Table 1, the locations of the suspected malignant lesions in each specimen were correlated with the MRI scans.
Statistical analysis was performed. The concordance rate between the robotic system tissue mapping and MRI scan results was 90.0% (54/60). The sensitivity and specificity of our robotic indentation system were 87.9% (29/33) and 92.6% (25/27), respectively. The PPV and negative predictive value were 93.5% (29/31) and 93.1% (27/29), respectively.
In recent years, extended or saturation biopsies have been performed in attempts to improve prostate cancer detection rates over systemic sextant biopsy.9 Djavan, et al.10 performed a prospective study on the pathologic cancer detection rates of first, second, third, and fourth biopsies, and found that the cancer detection rates were 22% (231/1051), 10% (83/820), 5% (36/737) and 4% (4/94), respectively. After taking into account the prostate volume in prostate cancer detection,9 Remzi, et al.9 proposed an optimal number of biopsy cores, based on the age of patients and the total prostate volume in patients with a PSA of 2 to 10 ng/mL. Despite a protocol-based systemic biopsies, their overall prostate cancer detection rate was only 36.7%.
Changes in the tissue elasticity may aid the identification of malignant cells as the cell integrity, and the intercellular matrix are pathologically changed in malignant transformation. Many measurements using mechanical devices have been performed to evaluate the properties of biological tissue using various techniques such as compressive pressure,11 indentation,12 aspiration,13 and shear strain. These measurements allow any change in tissue behavior and biology to be objectively and quantitatively measured.
Previous in vitro studies had been conducted to demonstrate the 'stiffness' in cancer tissue with dynamic indentation and showed differences in the elastic modulus of cancer tissues from normal ones. To 'visualize' tissue elasticity, ultrasound real-time elastography has been utilized in clinics. However, this colored visualization of elasticity is not an objective and quantitative measurement. In addition, the compressive strength is not constant as this procedure is done free-handed and this makes objective comparisons difficult, if not impossible. In a previous study, elasticity mapping of normal tissue and cancer tissue were developed with the numerical function and the nonlinear surface fitting method.8 This map served as our criteria for the diagnosis and localization of prostate cancer.
Our mechanical tissue mapping provides a quantitative and objective measure of suspected malignant lesions. With our robotic system, physicians can obtain objective mechanical and biological information about the suspected lesion, and perform more targeted prostatic biopsies as compared to DRE alone. The optimal number of needle biopsies during prostatic biopsies remains controversial.14 Transrectal ultrasonography (TRUS) guided systemic needle biopsy is associated with significant adverse effects, and many physicians have argued about the appropriate number of biopsy cores necessary for cancer detection. In addition, due to the technical limitations of TRUS guided biopsy, even experienced urologists may perform inaccurate biopsies of a targeted area. Our robotic system may serve as an useful adjunct in the screening for prostate cancer. As DRE findings are subjective and physician-dependent, this could lead to imprecise exchange of important clinical history and information between physicians.
To validate the accuracy of our robotic indentation system, we compared our mechanical tissue mapping with MRI scans. Suspected malignant lesions on the tissue mapping closely corresponded to those seen on MRI scans. We believe that our robotic system will be a very useful and novel adjunct when translated to clinical practice. Being an ex vivo study, this comes with the usual associated limitations. Ideally, we should have cross validated our tissue mapping with actual pathological location of malignant areas on histology. However, our institution pathologists do not routinely provide the precise (12 areas) information which we needed for this study. In addition, the geometry of the human prostate is not flat or smooth; one other limitation of our study will be that the forces applied and the reaction force will not be uniformly constant throughout the specimen, limiting the achievement of ideal results. However, we recreated a 3D model of the human pelvis with artificial bones and ligaments in an attempt to simulate the in vitro conditions as closely as possible.
Of all noninvasive anatomic imaging modalities, MR imaging is most suitable for evaluating the prostate, as it has an unparalleled ability to depict details of the prostate, due to its exquisite soft-tissue contrast.15 Preliminary evidence suggests that MRI directed transrectal ultrasonography-guided biopsies may improve cancer detection rates.16 Stoianovici, et al.17 presented a fully automated MRI compatible "stealth" robot which can perform prostate biopsy and radioactive seed implantation for brachytherapy.
Current modalities in screening and diagnosis of prostate cancer are still sub-optimal due to low sensitivities and specificities. Therefore, more specific and sensitive advanced diagnostic tools will go a long way in the diagnosis and treatment of this common cancer. Our robotic system may have a role in achieving a more accurate guided biopsy and delivery of focal therapy to malignant lesions.
Figures and Tables
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2011 (6-2011-0130).
References
1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011; 61:212–236.
2. Borden LS Jr, Wright JL, Kim J, Latchamsetty K, Porter CR. An abnormal digital rectal examination is an independent predictor of Gleason > or =7 prostate cancer in men undergoing initial prostate biopsy: a prospective study of 790 men. BJU Int. 2007; 99:559–563.

3. Gosselaar C, Roobol MJ, van den Bergh RC, Wolters T, Schröder FH. Digital rectal examination and the diagnosis of prostate cancer--a study based on 8 years and three screenings within the European Randomized Study of Screening for Prostate Cancer (ERSPC), Rotterdam. Eur Urol. 2009; 55:139–146.

4. Yossepowitch O. Digital rectal examination remains an important screening tool for prostate cancer. Eur Urol. 2008; 54:483–484.

5. Mettlin C, Lee F, Drago J, Murphy GP. The American Cancer Society National Prostate Cancer Detection Project. Findings on the detection of early prostate cancer in 2425 men. Cancer. 1991; 67:2949–2958.

6. Bozeman CB, Carver BS, Caldito G, Venable DD, Eastham JA. Prostate cancer in patients with an abnormal digital rectal examination and serum prostate-specific antigen less than 4.0 ng/mL. Urology. 2005; 66:803–807.

7. Park KK, Chung MS, Chung SY, Kim JH, Chung BH. Effects of post biopsy digital rectal compression on improving prostate cancer staging using magnetic resonance imaging in localized prostate cancer. Yonsei Med J. 2013; 54:81–86.

8. Ahn B, Lorenzo EI, Rha KH, Kim HJ, Kim J. Robotic palpation-based mechanical property mapping for diagnosis of prostate cancer. J Endourol. 2011; 25:851–857.

9. Remzi M, Fong YK, Dobrovits M, Anagnostou T, Seitz C, Waldert M, et al. The Vienna nomogram: validation of a novel biopsy strategy defining the optimal number of cores based on patient age and total prostate volume. J Urol. 2005; 174(4 Pt 1):1256–1260.

10. Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001; 166:1679–1683.

11. Rosen J, Brown JD, De S, Sinanan M, Hannaford B. Biomechanical properties of abdominal organs in vivo and postmortem under compression loads. J Biomech Eng. 2008; 130:021020.

12. Samur E, Sedef M, Basdogan C, Avtan L, Duzgun O. A robotic indenter for minimally invasive measurement and characterization of soft tissue response. Med Image Anal. 2007; 11:361–373.

13. Nava A, Mazza E, Furrer M, Villiger P, Reinhart WH. In vivo mechanical characterization of human liver. Med Image Anal. 2008; 12:203–216.

14. Scattoni V, Zlotta A, Montironi R, Schulman C, Rigatti P, Montorsi F. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol. 2007; 52:1309–1322.

15. Bonekamp D, Jacobs MA, El-Khouli R, Stoianovici D, Macura KJ. Advancements in MR imaging of the prostate: from diagnosis to interventions. Radiographics. 2011; 31:677–703.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download