Abstract
Purpose
Although neoadjuvant therapy has been accepted as a treatment option in locally-advanced gastric cancer, its prognostic value has been difficult to evaluate.
Materials and Methods
Seventy-four gastric cancer patients who underwent gastrectomy after neoadjuvant treatment were divided into two groups according to the pathologic response: favorable (ypT0) and others (ypT1-4). The clinicopathologic characteristics, predictive factors for pathologic response, and oncologic outcome were evaluated.
Results
Eleven patients (14.8%) demonstrated ypT0 and the remaining 63 patients (85.2%) were ypT1-4. Chemoradiotherapy (CCRTx) rather than chemotherapy (CTx) was the only predictive factor for a favorable pathologic response. Chemotherapeutic factors and tumor marker levels did not predict pathologic response. The 1-, 2-, and 3-year disease-free survivals were 83.4%, 70%, and 52.2%. The 1-, 3-, 5-year overall survivals were 88.5%, 67.5%, and 51.2%, respectively. Although a complete pathologic response (ypT0N0M0) was achieved in 7 patients, 28.6% of them demonstrated recurrence of the tumor within 6 months after curative surgery.
Although complete surgical removal represents the only curative treatment for gastric cancer, preoperative chemotherapy (CTx) and chemoradiotherapy (CCRTx) have been increasingly used over the past decades for advanced gastric cancer with the goals of tumor downstaging and increasing the rates of curative surgical resection and survival.
The benefits of preoperative CTx and CCRTx in gastric cancer have been widely reported in Western trials. For locally-advanced gastric cancer, the R0 resection rate was 70% to 80%, and the complete pathologic response rate was 15% to 30%.1-4 However, in Eastern countries, including Korea and Japan, the primary treatment for locally-advanced gastric cancer is surgical resection. Many surgeons prefer the aggressive or extended surgical resection to medical treatment and worry that patients can miss the chance for cure during the preoperative treatment due to disease progression. Therefore, preoperative treatment is not widely performed in Eastern countries, and data for oncologic outcomes in patients with neoadjuvant treatment are lacking. Therefore, postoperative pathologic stages are not able to exactly reflect the preoperative and initial tumor stage because of tumor regression and related histopathologic deformation.
In recent years, more patients have been diagnosed with locally-advanced gastric cancer who underwent gastrectomy after preoperative treatment such as CTx or CCRTx. The outcomes were mixed, demonstrating both excellent and poor pathologic responses. Therefore, we evaluated the data from gastric cancer patients who underwent surgical treatment after preoperative CTx or CCRTx to identify factors predictive of a favorable pathologic response and improved survival.
The surgical and pathologic data of 74 patients who underwent gastrectomy with lymph node dissection after preoperative CTx or CCRTx between 2000 and 2010 due to locally advanced gastric cancer were reviewed. These patients had advanced gastric cancer with regional lymph node metastasis and there was no distant metastasis in preoperative evaluation including endoscopic and radiologic imaging. Their preoperative treatment was done with the intent of neoadjuvant therapy.
Patients were divided into two groups according to the postoperative pathologic results: favorable response (ypT0) or others (ypT1-4). The ypT0 group included patients with no evidence of residual cancer in the stomach regardless of lymph node status. The ypT1-4 group included all patients with residual tumor in the stomach. All patients had histologically-confirmed adenocarcinoma in the stomach preoperatively.
The preoperative CTx regimen consisted of two or three of the following drug combinations: 5-fluorouracil based regimens (5-FU, capecitabine, S-1), leucovorin, platinum (cisplatin, oxaliplatin), taxol (taxotere, docetaxel), and irinotecan. The combination of two regimens included 5-FU with platinum, 5-FU with leucovorin, and irinotecan with platinum. For the combination of three regimens, 5-FU, platinum and taxol were used. Patients were divided into three groups according to the total number of cycles of preoperative CTx: 1) ≤3 cycles; 2) 4-6 cycles; and 3) >6 cycles. Patients received either CTx (n=55) or CCRTx (n=19), and there was no crossover between groups. The total preoperative radiotherapy dose was 4500 cGy.
Surgical treatment included standard or extended gastrectomy with lymph node dissection according to the guidelines of the Japanese Gastric Cancer Association.5
Because the standard extent of lymph node dissection in advanced gastric cancer is D2, most patients received D2 lymph node dissection. However, some patients with non-curative resection received D1+b lymph node dissection. Extended gastrectomy included resection of adjacent organs, such as the spleen, colon, pancreas, small bowel, and liver, in addition to the subtotal or total gastrectomy. R0 resection indicates a complete resection with no gross evidence of residual tumor. R1 resection refers to tumor involvement of the margins of the resected tissue when viewed microscopically, and R2 resection indicates that portions of visible tumor were not removed.
Clinicopathologic characteristics, such as sex, age, American Society of Anesthesiologists (ASA) score, tumor location, histologic type, number of chemotherapeutic regimens, number of chemotherapeutic cycles, and laboratory markers, were examined based on ypT status. Laboratory data included carcinoembryonic antigen, cancer antigen 72-4, cancer antigen, white blood cell count, neutrophil and lymphocyte count. The initial pretreatment values and preoperative values after CTx or CCRTx were checked. Surgical outcomes including the extent of resection, extent of lymph node dissection, operative time, and postoperative complications were also evaluated.
After R0 resection, the incidence of tumor recurrence and recurrence pattern were evaluated according to the ypT and ypN status. The tumor recurrence patterns were classified as lymphatic, peritoneal, hematogenous, and multiple recurrences. The evaluation of the pathologic stage was based on the 7th edition of the International Union Against Cancer Classification.6 The overall survival (OS) rate was calculated from the day of surgical resection until the time of death.
Statistical analysis was performed using SPSS® version 15.0 for Windows® (SPSS, Chicago, IL, USA). Categorical variables were analyzed using the chi-square or Fisher's exact test, and continuous data were analyzed using the Mann-Whitney U test. Data are presented as mean±standard deviation. OS curves were calculated in months based on the length of time between primary surgical treatment and final follow-up or death using the Kaplan-Meier method. The log-rank test was used to assess the statistical differences between variables. p-values <0.05 were considered significant.
Out of the 74 patients, 11 patients (14.8%) showed favorable response (ypT0) and had no residual cancer in the resected stomach. The residual tumor status, clinical features, type of preoperative therapy, and tumor characteristics are displayed in Table 1.
Age, sex, preoperative ASA score, tumor location, and histologic type were not predictive factors for a favorable pathologic response after CTx or CCRTx. In addition, the presence of adjacent organ invasion at the time of initial diagnosis noted on abdomen-pelvis computed tomography and/or endoscopic ultrasound was not predictive of pathologic response. The ypT0 rate was higher in patients with CCRTx than in CTx only (p=0.027). The incidence of a favorable response (i.e., ypT0) was 31.6% in the CCRTx group and only 9.1% in the CTx group. The number of preoperative chemotherapeutic regimens and total number of chemotherapy cycles were not associated with the pathologic response. The levels of the initial and preoperative tumor markers did not predict the pathologic response.
There were no significant differences in surgical outcomes, including the extent of resection, lymph node dissection, curability, operative time, number of retrieved lymph nodes, and postoperative hospital stay, between the ypT0 and ypT1-4 groups (Table 2). The ypT1-4 group was associated with a more advanced ypN status than the ypT0 group.
Four of the 11 patients in the ypT0 group underwent total gastrectomy. Seven patients in the ypT0 group exhibited ypN0, which indicated an approximately 9% (7 out of 74) rate of complete pathologic remission. In the ypT1-4 group, 9 patients (12.7%) could not undergo R0 resection: peritoneal seeding (n=8) and positive margins on resection (n=1). The mean number of retrieved lymph nodes was 39.0±17.7 in the ypT0 group and 38.0±17.3 in the ypT1-4 group. There were no significant differences in postoperative complications and the duration of hospital stay between the two groups.
Over the median 25-month follow-up, the 1-, 2-, and 3-year disease-free survival rates were 83.4%, 70.0%, and 52.2%, respectively. The 1-, 3-, and 5-year overall survival rates were 88.5%, 67.5%, and 51.2%, respectively. The survival curves were plotted for the ypT and ypN status (Figs. 1 and 2). The survival curve for ypN status reflected patients' prognoses better than ypT status. The modality of preoperative treatment (CCRTx vs. CTx) and the postoperative chemotherapy were not associated with patient survival.
As shown in Table 3, patients were divided according to the ypT and ypN status and evaluated for tumor recurrence. Among 7 patients with ypT0N0, 2 (28%) patients showed tumor recurrence 4.7 months and 6.1 months after radical gastrectomy. The 2 patients with tumor recurrence were among the 5 patients who did not receive postoperative CTx. Neither of the 2 patients who received postoperative CTx showed tumor recurrence in patients with ypT0N0. Among the 3 patients with ypT0N1, only the one patient who did not receive postoperative CTx had a tumor relapse.
None of the patients with ypT1N0 (n=3) showed recurrence, and only 1 of 5 patients with ypT2N0 exhibited tumor recurrence. Recurrence rates were 33% to 50% in patients with ypT3 according to the ypN status. Recurrence rates ranged from 25% to 100% in those patients with ypT4. Tumor recurrence following gastrectomy generally occurred within a few months in most patients.
Gastrectomy with D2 lymph node dissection and extended surgery for resection of involved organs is the current standard of care for advanced gastric cancer.5,7,8 In recent years, a multidisciplinary treatment approach, including perioperative CTx, radiotherapy, and targeted therapy, has emerged for advanced gastric cancer, resulting in increased curability and improved survival. Recent studies have suggested that neoadjuvant CTx in patients with unresectable, locally-advanced or metastatic gastric cancer can offer a chance for curative resection and improved survival.9,10
In the present study, we focused on identifying clinical factors predictive of an excellent response to preoperative CTx or CCRTx. As shown in Table 1, several clinicopathological factors were analyzed to find the predictive factor for favorable response to preoperative treatment. We found that histological type, preoperative chemotherapeutic regimens, and tumor markers were not associated with a favorable response. The only factor related to the favorable response was the type of preoperative treatment; either CTx or CCRTx. Although our curative resection rate was 87.8% (65 out of 74 patients), which is similar to other studies,2-4 our rate of complete pathologic response was only 9.4% (7 out of 74 patients). Because this study was retrospective in nature, the chemotherapeutic protocols varied among the patients, which might explain why our 9.4% complete pathologic response rate was lower than the reported 15% to 30% in previous prospective studies.1,2,4 Overall, we were able to identify ypT0 patients as those patients likely to have a favorable response to preoperative treatment regardless of ypN status.
Neoadjuvant CCRTx was more effective than neoadjuvant CTx for tumor regression in gastric cancer. The favorable response rate (ypT0) was 31.6% after CCRTx and only 9.1% after CTx. In addition, the incidence of ypN0 was 47.4% (9 patients) after CCRTx and 27.3% (15 patients) after CTx. The impact of preoperative CTx or CCRTx in esophagogastric junction (EGJ) cancer was observed in several Western studies.11,12 Additionally, one phase III study comparing CTx and CCRTx showed a higher complete pathologic response rate in the preoperative CCRTx group than in the preoperative CTx group.13 Although these Western studies focused on esophageal and EGJ cancer, the superiority of adding radiation therapy to preoperative CTx may be applied to gastric cancer, too.
However, in this retrospective study, the initial tumor status and the treatment efficacy for CCRTx and CTx in histologic tumor regression of gastric cancer could not be directly compared. Moreover, because the preoperative treatment of the neoadjuvant intent was not routine and the indications of preoperative CCRTx or CTx were not well established in our institute, CTx or CCRTx was done arbitrarily according to the surgeons' or oncologists' decision in locally far advanced gastric cancer. Therefore, our data cannot offer conclusive evidence. It can only provide some clues for achieving better survival in advanced gastric cancer, which should be solved by more prospective studies.
The prognosis of patients with a favorable response was poorer than anticipated in our results, because two of our seven patients (28.6%) with complete pathologic response experienced tumor recurrence within a short time. These two patients did not receive adjuvant CTx; however, two patients with ypT0N0 who received adjuvant treatment did not experience tumor recurrence (Table 3). When the tumor was in the advanced stage, despite neoadjuvant treatment, the prognosis was very poor. Tumor recurrence developed rapidly, with the majority recurring within 12 months even after R0 resection. Therefore, postoperative adjuvant treatment may be necessary regardless of the surgical pathology stage. Because of the small number of patients, the survival analysis at each stage had some limitations. The rationale for adjuvant CTx in patients with complete pathologic remission is not conclusive; however, this study may offer clues toward improvement in the treatment of advanced gastric cancer with neoadjuvant therapy.
This retrospective study design resulted in several limitations, including the inability to strictly evaluate the initial tumor stage before neoadjuvant treatment and the lack of consistency with respect to the diagnostic modalities and neoadjuvant treatment protocols. It was difficult to determine whether patient survival was better represented by the initial tumor stage or the final pathologic tumor stage. Additionally, the degree of histologic tumor regression by neoadjuvant treatment was not evaluated in all patients. These drawbacks would be overcome with a prospective randomized study.
In summary, concurrent CCRTx rather than CTx alone appeared to be more effective with respect the pathologic response in locally-advanced gastric cancer and postoperative nodal status appeared to reflect patients' prognoses better than ypT status. Although favorable pathologic response by neoadjuvant treatment was achieved, survival was not as good even after radical surgery. Overall, these results suggest the survival benefit of neoadjuvant treatment regardless of the fact that resectability remains controversial.
Figures and Tables
Table 1
Comparison of Preoperative Clinical, Therapeutic, and Tumor Features
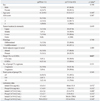
ASA, American Society of Anethesiologists; CA, cancer antigen; CEA, carcinoembryonic antigen; CT, computed tomography; CTx, chemotherapy; CCRTx, chemoradiotherapy; EUS, endoscopic ultrasound.
*Chi-square.
†Initial adjacent organ invasion was evaluated by abdomen-pelvis CT scan and/or EUS before CTx or CCRTx.
‡Mann-Whitney U test, mean±standard deviation. Significant values are indicated in bold face. Values in parentheses are percentages.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2011-0011301).
References
1. Rohatgi PR, Mansfield PF, Crane CH, Wu TT, Sunder PK, Ross WA, et al. Surgical pathology stage by American Joint Commission on Cancer criteria predicts patient survival after preoperative chemoradiation for localized gastric carcinoma. Cancer. 2006; 107:1475–1482.

2. Ajani JA, Mansfield PF, Janjan N, Morris J, Pisters PW, Lynch PM, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004; 22:2774–2780.

3. Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011; 253:934–939.

4. Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005; 23:1237–1244.

6. Sobin LH, Wittekind CH. International Union Against Cancer (UICC). TNM Classification of Malignant Tumours. 2009. 7th ed. New York: Wiley.
7. An JY, Cheong JH, Hyung WJ, Noh SH. Recent evolution of surgical treatment for gastric cancer in Korea. J Gastric Cancer. 2011; 11:1–6.

8. Lee JH, Kim KM, Cheong JH, Noh SH. Current management and future strategies of gastric cancer. Yonsei Med J. 2012; 53:248–257.

9. Sym SJ, Chang HM, Ryu MH, Lee JL, Kim TW, Yook JH, et al. Neoadjuvant docetaxel, capecitabine and cisplatin (DXP) in patients with unresectable locally advanced or metastatic gastric cancer. Ann Surg Oncol. 2010; 17:1024–1032.

10. Lordick F, Stein HJ, Peschel C, Siewert JR. Neoadjuvant therapy for oesophagogastric cancer. Br J Surg. 2004; 91:540–551.

11. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006; 355:11–20.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download