Abstract
Purpose
Phosphatidylinositol 3-kinases/AKT pathway plays a pivotal role in hepatocellular carcinoma (HCC). Mutant PIK3CA, encoding the p110a catalytic subunit, stimulates the AKT pathway and promotes cell growth in various cancers. PIK3CA mutation rate has been usually reported as low frequency (<5%) in HCC except one report from Korea with 35.6%. Therefore, we investigated the frequency of PIK3CA mutations in Korean HCC patients.
Materials and Methods
We sequenced exons1, 3, 4, 6, 7, 8, 9, 19 and 20 of PIK3CA in 268 HCC tumor tissue samples by Sanger method and pyrosequencing assay.
Results
In this study, the mutations were not detected in exons3, 6, 8, and 19, and detected 1 at unknown SNP in exon1 and exon4, 2 at unknown SNP in exon7, 2 at unknown SNP in exon20. However, 1 at unknown SNP, 1 at G1635T and surprisingly all samples at A1634Cin exon9 were detected by Sanger method. Additional experiments with normal tissue, cloning experiments and a pyrosequencing assay revealed that the double peak at A1634C of exon9 is a pseudogene, not true mutation. The mutations found in this study were all different and small numbers, therefore, we cannot conclude specific relationship between clinical characteristics of HCC and mutation of PIK3CA.
With an annual incidence of >560000 deaths, hepatocellular carcinoma (HCC) is globally the sixth most common malignancy and the third leading cause of cancer-related mortality.1 Liver cancer accounts for 4% of all cancers; >70% of all liver cancers occur in Asia, and the incidence of liver cancer in the Far East countries, including Korea, China, and Japan is very high.2 Recent studies demonstrated that genetic alternations and signaling pathways are one of the determinants for the development of cancer.3-5 Phosphatidylinositol 3-kinases (PI3Ks) are expressed as heterodimers of p110 catalytic subunits, and p85 regulatory subunits interact with phosphatidylinositol-3-phosphate at the membrane and catalyze the phosphorylation of AKT, which activates the downstream signaling pathway.6 PI3K/AKT/mTOR pathway plays a pivotal role in HCC and is activated in 30-50% of HCC cases.7,8 Mutant phosphoinositide-3-kinase [catalytic alpha polypeptide (PIK3CA), encoding the p110a catalytic subunit] stimulates the AKT pathway and promotes cell growth in various types of cancer,9 including breast, colon, brain, gastric and lung.10-12 Moreover, over 75% of mutations occur in the helical and kinase domains and these locations are likely to function as an oncogene in human cancer.7 The mutation of PIK3CA in HCC has been investigated, where most reports cited low frequency of mutation (0-3.5%) except for a Korean report (35.6%),13 0% (0/47) in Japan,14 3.5% (2/57) in France,15 2% (1/50) in Switzerland,16 1.1% (1/90) in China17 and 28% (18/65) in South Italy.18 Therefore, we investigated whether the mutation of PIK3CA varies depending on races or regions, employing 268 Korean HCC patients.
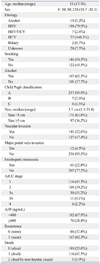
A total of 268 consecutive patients who had undergone surgery for HCC at Samsung Medical Center from July 2000 and May 2006 were included in this study. All cases were histopathologically confirmed after operation. This study was performed with appropriate Institutional Review Board approval. The clinical characteristics of the subjects are shown in Table 1. Genomic DNA was extracted from tumors and normal tissues obtained from fresh frozen tissue using QIAamp DNA Mini Kit® (Qiagen, Hilden, Germany).
We designed polymerase chain reaction (PCR) primers using the Primer 3 program. Primer pairs were designed to produce about 500 base pairs by PCR, and their TMs ranged between 59℃ and 62℃. PCR was performed with 20 ng of genomic DNA as the template in a 30 µL reaction mixture by using a EF-Taq (SolGent, Daejeon, Korea) as follows: activation of Taq polymerase at 95℃ for 2 minutes, 35 cycles of 95℃ for 1 minute, 55℃, and 72℃ for 1 minute each were performed, finishing with a 10-minute step at 72℃.
The amplification products were purified with a multiscreen filter plate (Millipore Corp., Bedford, MA, USA). Fragments were ligated into a pTOP TA vector using a MGTM TOPcloner TA kit (Enzynomics, Daejeon, Korea). Sequencing reaction was performed using a PRISM BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). The DNA samples containing the extension products were added to Hi-Di formamide (Applied Biosystems, Foster City, CA, USA). The mixture was incubated at 95℃ for 5 min, followed by 5 min on ice and then analyzed by ABI Prism 3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA).
Mutation analysis was performed by pyrosequencing on the PyroMark Q24 (Qiagen, Germantown, MD, USA). First, PCR products were immobilized on streptavidin-coated beads and denatured to produce single-stranded products. Pyrosequencing was performed using the PyroMark Gold Q24 reagent (Qiagen, Germantown, MD, USA), according to the manufacture's protocol. Exon9 primers for the forward: 5'(Biotin)-ATTTCTACACGAGATCCTCTCTCT-3'. Reverse primer: 5'-CCATTTTAGCACTTACCTGTGAC-3' sequencing primer: 5'-TAGAAAATCTTTCTCCTG-3'. Sequencing analysis was performed using PyroMark Q24 version 1.0.10 software in the allele quantification analysis mode.
To investigate the frequency of PIK3CA mutations in Korean patients with HCC, we sequenced the exon1, 3, 4, 6, 7, 8, 9, 19 and 20 of PIK3CA in 268 HCC samples. In these experiments, mutations were not detected in exon3, 6, 8, or 19 in any of the HCC samples and detected 1 of 268 at unknown SNP (G278A) in exon1 and at unknown SNP (C848G) in exon4, 2 of 265 at unknown SNP (C1306A) and 1 of 265 at unknown SNP (C1373A) in exon7, 1 of 262 at unknown SNP (T2970A) and SNP (G3026A) in exon20 (Fig. 1A). However, in exon9 we detected 1 of 266 at unknown SNP (C1629T), 1 of 268 at G1635T (E545D) and surprisingly we observed a nucleotide alteration in 266 of 266 at A1634C (E545A) (Fig. 1B).
We investigated whether two peaks at nucleotide position 1634 in exon9 was a true mutation or not. However, PCR products from 52 normal tissues also had all nucleotides changed in exon9. For the exact detection, PCR amplification and subsequent cloning with 5-7 colonies picked both forward and reverse direction in 50 HCC tumor samples and 10 colonies picked forward direction in 34 matched normal tissue samples. Furthermore, sequencing of the PCR products revealed A1634C mutation in all samples in each 5-10 clone from the same sample (supplementary data). However, we need to confirm whether the nucleotide change 1634 A→C was real because chromosome 22 has C at 1634 and C at 1658 sequencing. In order to confirm pseudogene results and sequencing methods, we performed pyrosequencing assay with HCC tumor tissues, and found that all 268 samples showed A at nucleotide 1634 (Fig. 2). Additional experiments with normal tissue, cloning experiments and pyrosequencing assay revealed that codon 545 of exon9 was all wild type. With these results, we analyzed the sequence of PIK3CA. Since the mutations found in our study were all different and so small numbers, we cannot conclude any specific relationship between mutation in the exon of PIK3CA and clinical characteristics of HCC (Table 2).
PIK3CA has been shown to be mutated in various tumors and recognized as a possible therapeutic marker. More than 70% of all liver cancers occur in Asia, while the frequency of mutation varied among several reports from Asia. If PIK3CA mutation of HCC in Korean patients is specifically higher as reported by Lee, et al.13 who investigated 73 HCC tissues, it might be a new target of Korean HCC. For this reason, confirmatory study is needed, including more tissues.
We sequenced exons1, 3, 4, 6, 7, 8, 9, 19 and 20 of PIK3CA in 268 HCC tumor tissues. The mutations were not detected in exons 3, 6, 8, and, 19 and detected 1 of 268 at 278 G→A in exon1 and at 848 C→G in exon4, 2 of 265 at 1306 C→A and 1 of 265 at 1373 C→A in exon7, each of 262 at 2970 T→A and 3026 G→A in exon20. However, 1 of 266 was detected at 1629 C→T, 1 of 268 at 1635 G→T and 266 of 266 at 1634 A→C in exon9. For validation with normal matched tissues, we found that 1634 A→C also occurred.
Through cloning and pyrosequencing methods, the double peak at A1634C of exon9 was found not to be a mutation, but occurred as a result of gene duplication on 22q11 partial region. Cat Eye Syndrome is a hereditary disease characterized by ocular colobomata, anal atresia, congenital heart defects, and mental retardation, and mutations are located on human chromosome 22q11.19 We confirmed that the nucleotide sequence of exon9 has a highly homologous match rate (97%) on chromosome 22 in NCBI BLAST as Tanaka, et al.14 previously mentioned. A1634C was found in all samples. For more exact analysis, we analyzed 10 colony sequencing per each sample through cloning experiments, and found 1633-GAGCAGGAGAAAGATTTTCTATGGAGTCACAG in PIK3CA and 1633-GCGCAGGAGAAAGATTTTCTATGGACCACAG in chromosome 22. Yet, when the sequence occurred from C or G at position 1634 to GT at position 1658, this was a mutation. However, there might be different results as wild type and pseudogene mutation depending on clones in one sample; if there is a mutation in one clone even within the same sample, it will be considered to be a mutation. As the number of clones increase, the probability of mutations in all samples will be increasing.
This analysis was not optimal because primers are likely to amplify both genes, PIK3CA exon9 and chromosome22. For analysis of nucleotide 1634, pyrosequencing is more efficient at the amplification step in heterogeneous samples. Detection of codon 545 of PIK3CA is well suited to be examined by pyrosequencing as Baker, et al.20 mentioned recently. Following Baker, et al.'s methods, we confirmed again that all samples were wild type at codon 545.
In this study, unknown SNP G278A, C848G, C1306A, C1373A, C1629T, T2970A, G3026A were found, however, only 8 cases of 268 HCC tumor tissues were found to be mutated. Even though we found low mutation frequency in our samples, amplification or mutation of PIK3CA has been reported in various cancer types, and the question of whether PIK3-AKT pathway is activated in HCC remains still controversial. Nevertheless, it is highly possible that this gene is a promising molecular target for cancer treatment. As nucleotide1634 in exon9 has sequence very similar to chromosome22, pyrosequencing assay for excluding pseudogene interference appears to be more proper for detecting mutation.
In conclusion, our study suggests that the rate of PIK3CA mutation in the Korea population is in fact similar to the rates seen elsewhere in the world.
Figures and Tables
 | Fig. 1Sequencing results of PIK3CA gene. (A) Electropherograms show the SNP in each exon in 268 HCC samples. (B) Electropherograms show the nucleotide sequences of the genomic DNA from red box, indicating double peak at 1634 of exon9 in 268 HCC samples. HCC, hepatocellular carcinoma. |
ACKNOWLEDGEMENTS
This study was supported by the Samsung Biomedical Research Institute Grant, #C-A9-236.
Dr. SY Rha's research was supported by the Public Welfare & Safety research program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2010-0020841).
References
1. GLOBOCAN. 2008 [Internet]. accessed on 2011 June 28. Available at: http://globocan.iarc.fr.
2. Korean National Cancer Center [Internet]. accessed on 2011 April 1. Available at: http://www.ncc.re.kr.
3. Marra M, Sordelli IM, Lombardi A, Lamberti M, Tarantino L, Giudice A, et al. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: an overview. J Transl Med. 2011; 9:171.

4. Caraglia M, Giuberti G, Marra M, Addeo R, Montella L, Murolo M, et al. Oxidative stress and ERK1/2 phosphorylation as predictors of outcome in hepatocellular carcinoma patients treated with sorafenib plus octreotide LAR. Cell Death Dis. 2011; 2:e150.

5. Prete SD, Montella L, Caraglia M, Maiorino L, Cennamo G, Montesarchio V, et al. Sorafenib plus octreotide is an effective and safe treatment in advanced hepatocellular carcinoma: multicenter phase II So.LAR. study. Cancer Chemother Pharmacol. 2010; 66:837–844.

7. Mínguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol. 2009; 25:186–194.

9. Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004; 3:1221–1224.

10. Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004; 304:554.

11. Lai YL, Mau BL, Cheng WH, Chen HM, Chiu HH, Tzen CY. PIK3CA exon 20 mutation is independently associated with a poor prognosis in breast cancer patients. Ann Surg Oncol. 2008; 15:1064–1069.

12. Miyaki M, Iijima T, Yamaguchi T, Takahashi K, Matsumoto H, Yasutome M, et al. Mutations of the PIK3CA gene in hereditary colorectal cancers. Int J Cancer. 2007; 121:1627–1630.
13. Lee JW, Soung YH, Kim SY, Lee HW, Park WS, Nam SW, et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005; 24:1477–1480.

14. Tanaka Y, Kanai F, Tada M, Asaoka Y, Guleng B, Jazag A, et al. Absence of PIK3CA hotspot mutations in hepatocellular carcinoma in Japanese patients. Oncogene. 2006; 25:2950–2952.

15. Boyault S, Rickman DS, de Reyniés A, Balabaud C, Rebouissou S, Jeannot E, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007; 45:42–52.

16. Riener MO, Bawohl M, Clavien PA, Jochum W. Rare PIK3CA hotspot mutations in carcinomas of the biliary tract. Genes Chromosomes Cancer. 2008; 47:363–367.
17. Li X, Zhang Q, He W, Meng W, Yan J, Zhang L, et al. Low frequency of PIK3CA gene mutations in hepatocellular carcinoma in Chinese population. Pathol Oncol Res. 2012; 18:57–60.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download