Abstract
Purpose
Recurrence rate is considered a better measure of clinical outcomes after thymoma resection than overall survival due to the indolent behavior of thymomas. This study was designed to determine predictors of recurrence after thymoma resection.
Materials and Methods
A single-institution, retrospective study was performed, including 305 patients who had undergone thymoma resection between 1986 and 2009.
Results
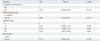
Among 305 patients, recurrence was observed in 41 patients (13.4%). The recurrence rates were 0% (0/19), 6.3% (4/63), 4.2% (2/48), 18.6% (11/59) and 20.7% (24/116) for type A, AB, B1, B2 and B3 tumors, respectively. The recurrence rate according to Masaoka stage was 6.1% (8/132), 11.4% (13/114), 26.8% (11/41) and 50.0% (9/18) for stages I, II, III and IV, respectively. After univariate analysis, completeness of resection (R0 versus R1), World Health Organization (WHO) histologic type (A, AB, B1 versus B2, B3), Masaoka stage, and size of tumor (<8 cm versus ≥8 cm) demonstrated significant differences with freedom from recurrence. Upon multivariate analysis, Masaoka stage was the only independent predictor of recurrence.
Thymomas are tumors that originate from thymic epithelial cells, demonstrating organotypic features. They are the most common mediastinal tumor, accounting for approximately 20% of all mediastinal masses and up to 50% of all anterior mediastinal masses.1 However, because thymomas involve a wide spectrum of histological, biological and oncological characteristics, not only are there no uniform guidelines concerning the management thereof, but outcomes after management and subsequent prognoses have also not been well established. Thus, the International Thymic Malignancy Interest Group (ITMIG) has attempted to establish standard definitions and policies for thymoma management. ITMIG has suggested that freedom-from-recurrence is a better measure than survival in patients who have successfully undergone curative-intent treatment.2 Their reasoning for this was that thymomas progress slowly, and thus, many patients die of causes unrelated to the thymoma. In actuality, only -50% of fatalities are due to the thymoma or the treatment thereof. Approximately, 20% of deaths are due to myasthenia gravis, and 10% result from autoimmune disorders associated with thymoma. The remaining deaths have been attributed to unrelated conditions, including other malignancies.1 Accordingly, recurrence may more accurately reflect clinical outcomes after resection than survival.
Several factors, such as Masaoka stage, World Health Organization (WHO) histological type, completeness of resection, and tumor size have been shown to be prognostic factors influencing survival after thymoma resection.3-8 Compared to the studies on survival, only a few reports have addressed predictive factors of recurrence.5,9
Therefore, we conducted a retrospective study to determine predictors of recurrence after thymoma resection.
The Institutional Review Board of Yonsei University College of Medicine approved this retrospective study. The need for individual consent from patients whose records were evaluated was waived because individuals were not identified in the study.
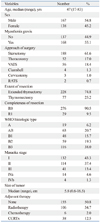
Four hundred and eleven consecutive patients underwent surgery for a thymic epithelial tumor at our institute between January 1986 and December 2009. Of those, we excluded patients with an undetermined WHO histologic type (n=19) due to the unavailability of specimen slides or a total infarcted tumor; patients with type C thymoma (n=68); and patients who only underwent an open biopsy or R2 resection (n=19). The medical records of the remaining 305 patients were reviewed retrospectively.
Pathological results were confirmed by an experienced pathologist (W.I. Yang) who was blind to the clinical data. The thymomas were classified into histological types A, AB, B1, B2 and B3, according to the WHO classification system.7 When a tumor exhibited mixed histologic types, the tumor was classified as the most histologically aggressive type observed. For example, when the tumor had both B2 and B3 components, the tumor was classified as type B3. Tumor stage was classified into I, II, III, IVa, IVb, following the Masaoka classification system;8 stage was determined by review of surgical records and pathological reports.
Extended thymectomy was defined as the resection of the entire thymus and mediastinal fat tissue between both phrenic nerves. Thymomectomy was defined as the resection of the thymoma along with the surrounding fatty tissue, leaving residual thymic tissue.10
Complete thymectomy has been widely supported for thymoma resection. However, in some patients without myasthenia gravis, we performed thymomectomy at the discretion of the surgeon, depending on the location and size of thymoma. This policy was supported by the results of a previously published report by our institution in which no difference in survival between extended thymectomy and thymomectomy was noted.10
Completeness of resection was categorized as follows: R0 resection indicated no residual tumor tissue; R1 resection indicated microscopic residual tumor tissue; and R2 resection indicated macroscopic residual tumor tissue; R2 patients were excluded from this study. Patients with stage IVa tumors that were completely removed, including all of the involved pleural and pericardial lesions, were classified as a R0 resection.
Our strategies for adjuvant therapy were as follows: no adjuvant therapy for stage I thymomas, radiotherapy for invasive or incompletely resected thymomas, and chemotherapy (with or without radiotherapy) for Masaoka stage IV thymomas. However, because this strategy was not standardized for the entire study period, the strategy for adjuvant therapy was patient specific according to each surgeon's preference.
Adjuvant chemotherapy was generally performed according to the ADOC regimen (doxorubicin, cisplatin, vincristine, and cyclophosphamide) for a total of 6 cycles every 3-4 weeks.
Radiotherapy was applied at a median dose of 5040 cGy (180 cGy/fraction, range, 4500 to 6300 cGy) for 5-6 weeks.
All patients were followed up at the outpatient clinic at 3-month intervals for the first year, at 6-month intervals for the subsequent year, and yearly for the next 3 years. A physical examination and chest radiography were performed upon each visit to the outpatient clinic. A chest computed tomography (CT) was performed at 6-month intervals for the first 2 years and at 1-year intervals for the subsequent 3 years. However, there was some variation in the follow-up procedure that was followed depending on the stage and histology of each tumor, as well as the surgeon's preference. After 5 years, patients were followed up at our neurology or oncology department for any prevailing medical problems. The data for the nine patients who did not attend follow-up sessions were obtained by direct telephone contact for this study. Follow-up for this study was complete up to December 2010.
Recurrence was divided into three categories according to the definition proposed by ITMIG.2 Local recurrence was defined as disease appearing in the anterior mediastinum or tissues immediately contiguous with the resected thymoma (i.e., pleural recurrence in the area of a previously resected stage IVa tumor, cervical lymph node adjacent to a previously resected thymoma). Regional recurrence was defined as intrathoracic recurrence (i.e., pleural and pericardial nodules). Distant recurrence included extrathoracic recurrence and intraparenchymal pulmonary nodules.
Comparative analyses to identify differences in patient and tumor characteristics were performed using the χ2 test. Fisher's exact test was conducted to analyze categorical variables, and Student's t-test was used for continuous variables. Freedom from recurrence was calculated using the Kaplan-Meier method, and statistical differences in recurrence were determined using the log-rank test. Recurrence of thymoma was considered as an event. All deaths without recurrence were considered as censored observations. The recurrence free period after the resection was calculated from the date of the resection to the date of the last follow-up or of recurrence diagnosis. For multivariate analysis, a Cox proportional hazards regression model was performed. A p-value of less than 0.05 was considered statistically significant. All statistical analysis was performed using SPSS software version 18.0 for Windows (Statistical Package for Social Science, SPSS Inc., Chicago, IL, USA).
The characteristics of the patients and tumors are listed in Table 1.
Recurrence was observed in 41 (13.4%) of the 305 patients. The median time to recurrence was 52 months (range, 6 to 234 months). Six patients (14.6%) demonstrated local recurrence, 24 (58.5%) regional recurrence, 5 (12.2%) distant metastasis, and 6 patients (14.6%) demonstrated overlapped recurrence (4 local/regional, 1 local/distant, and 1 regional/distant). Distant metastases were found in 7 patients: four lung, two liver, and one abdominal lymph node metastases.
The recurrence rates were 0.0% (0/19), 6.3% (4/63), 4.2% (2/48), 18.6% (11/59) and 20.7% (24/116) for type A, AB, B1, B2 and B3 tumors, respectively. The recurrence rates according to Masaoka stage were 6.1% (8/132), 11.4% (13/114), 26.8% (11/41), 50.0% (7/14) and 50.0% (2/4) for stages I, II, III, Iva and IVb, respectively.
Recurrence rates according to WHO histological type and Masaoka stage are shown in Table 2.
After a median follow-up of 67 months (range, 2 to 241 months), 239 (78.4%) patients were alive and well, 24 (7.9%) were alive with recurrence and 42 (13.8%) had died. Of those who died, 22 (52.3%) died of thymoma or treatment related causes, and the remaining 20 patients died due to other causes. The other causes of death were as follows: seven from unknown causes, four from cardiovascular disease, three from respiratory failure due to myasthenia gravis, two from a traffic accident, one from liver cirrhosis, one from primary lung cancer, one from hepatocellular carcinoma and one from delayed mediastinitis.
The overall survival rates at 5, 10 and 15 years were 89.8, 82.9 and 74.9%, respectively (Fig. 1).
Recurrence rate according to the identified variables and freedom from recurrence over time are shown in Table 3.
In the univariate analysis, no significant differences in freedom from recurrence were observed according to age, sex, presence of myasthenia gravis and extent of resection. In cases of R1 resection, higher WHO histological type (B2, B3), advanced Masaoka stage, larger thymoma size (≥8 cm) and no adjuvant therapy, a higher risk of recurrence was observed. The differences in freedom from recurrence between these groups are shown in Fig. 2.
In multivariate analysis after adjusting for all the significant variables of the univariate analysis, no significant relationships were found.
However, as adjuvant therapy was shown to be significantly correlated with Masaoka stage, a separate Cox regression analysis was performed excluding adjuvant therapy. In this multivariate analysis, Masaoka stage III and IV were shown to be independent predictors of tumor recurrence (stage III, hazard ratio, 3.912; 95% confidence interval, 1.302 to 11.751; p=0.015, stage IV, hazard ratio, 5.236; 95% confidence interval, 1.407 to 19.482; p=0.014) (Table 4).
Our study showed that WHO histological type, Masaoka stage, and size of tumor are associated with recurrence after thymoma resection. In addition, Masaoka stage was shown to be the only independent predictor of recurrence after thymoma resection. These findings corroborate many previous studies concerning prognostic factors of survival after thymoma resection.5,9
According to WHO histological type, histologic type A, AB and B1 thymomas demonstrated a lower risk of recurrence than types B2 and B3 in this study. Similar to these results, Strobel, et al.11 reported that types A, AB and B1 behaved in a benign fashion, while types B2 and B3 behaved malignantly. Okumura, et al.3 also reported that type B2 and B3 tumors were more malignant in nature, in terms of tumor recurrence, compared with types A, AB and B1.
Regarding stage, Detterbeck1 reported that average recurrence rates were low for Masaoka stage I tumors (3%), but increased progressively to 11 and 30% for stage II and III tumors, respectively. Our observations corroborated these findings and showed that Masaoka stage was the only independent predictor of recurrence after thymoma resection.
Wright12 suggested that a tumor size of 8 cm or larger was an independent risk factor of recurrence, and that large masses in which invasiveness is not clearly observable on CT are likely to be treated as stage III tumors. Our results also showed that a tumor size of 8 cm or larger was significantly associated with recurrence.
In our study, the most frequently observed histological subtype was B3 (38%), followed by AB (20.7%), B2 (19.3%), B1 (15.7%) and type A (6.2%). However in a study by Margaritora, et al.,13 which analyzed 317 patient (including some with WHO type C tumors), type B2 tumors were most frequently observed (57.5%), followed by types B1 (19.2%), AB (9.5%), B3 (8.2%) and type A (3.5%). This difference could have been due to their high incidence rate of myasthenia gravis (87%) and differences in our classification system, which classified mixed type tumors as the higher component type. Strobel, et al.11 reported on 28 type B3 tumors (12.3%) out of 228 patients and B2+B3 tumors in 42 patients (18.4%) and by reclassifying these combined tumors according to our classification, B3 tumors would have been recorded in 71 patients (31%).11 Their distribution of histological types was similar to ours.
In our study, stage I, II, III and IV tumors comprised 43, 37, 13 and 6% of all thymomas, respectively. The study of Margaritora, et al.13 exhibited a similar distribution of 42, 32, 22 and 4%, respectively.
In our study, there was no operative mortality. Operative mortality in this study was lower than that in other reports,4,6 potentially due to exclusion of type C thymomas. Thymoma-related or treatment-related deaths accounted for 52% (22 of 42) of all mortality. This result was consistent with other reports,1,14 and supports our current stance that recurrence is a better measure of clinical outcome after thymoma resection than overall survival.
There are several limitations to our study. First, our conclusion that Masaoka stage is the most important predictor of recurrence is not a novel finding because many studies have reported on the importance of Masaoka stage in the prognosis of thymoma. However, we focused on freedom from recurrence as a measurement of outcomes after resection as recommended by ITMIG. Second, our study was a retrospective study performed at a single institution covering a follow-up period over which the operational approach, treatment modality and follow-up policy were not uniform. Thus, this study's results may be influenced by intrinsic bias. Third, Masaoka stage is a surgical staging system; therefore, it is not reliable in preoperative settings. Further studies are needed to determine preoperative predictive factors. Fourth, ITMIG recommends freedom-from-recurrence as the best measurement of clinical outcome for patients who have undergone an R0 resection.2 We included patients who had undergone an R1 resection because these patients received adjuvant radiotherapy after the resection, and thus, were considered to have successfully undergone curative-intent treatment. Finally, our follow-up method of five years after thymoma resection was not appropriate to confirm recurrence. We included nine patients who were followed-up by phone call as having no recurrence; however, because we were not able to check their medical status, we were not able to confirm their reports. Additionally, for patients followed-by the neurology department, they did not undergo regular chest CTs; therefore, recurrence may have been missed resulting in an underestimated recurrence rate.
In conclusion, WHO histologic type, Masaoka stage and size of tumor were shown to be associated with recurrence after thymoma resection. Particularly, Masaoka stage was the only independent predictor of recurrence after thymoma resection.
Figures and Tables
 | Fig. 1The overall survival of patients who underwent thymoma resection. The 5-, 10- and 15-year survival rates were 89.8, 82.9 and 74.9%, respectively. |
 | Fig. 2Freedom from recurrence curves according to completeness of resection (A), WHO histological classification (B), Masaoka stage (C), size of tumor (D) and adjuvant therapy (E). |
References
1. Detterbeck FC. Evaluation and treatment of stage I and II thymoma. J Thorac Oncol. 2010; 5:10 Suppl 4. S318–S322.

2. Huang J, Detterbeck FC, Wang Z, Loehrer PJ Sr. Standard outcome measures for thymic malignancies. J Thorac Oncol. 2010; 5:2017–2023.

3. Okumura M, Ohta M, Tateyama H, Nakagawa K, Matsumura A, Maeda H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer. 2002; 94:624–632.

4. Rea F, Marulli G, Girardi R, Bortolotti L, Favaretto A, Galligioni A, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg. 2004; 26:412–418.

5. Blumberg D, Port JL, Weksler B, Delgado R, Rosai J, Bains MS, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg. 1995; 60:908–913.

6. Regnard JF, Magdeleinat P, Dromer C, Dulmet E, de Montpreville V, Levi JF, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg. 1996; 112:376–384.

7. Rosai J, Sobin LH. Histological typing of tumours of the thymus. WHO International histological classification of tumours. 1999. 2nd ed. New York: Springer Verlag.
8. Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981; 48:2485–2492.

9. Wright CD, Wain JC, Wong DR, Donahue DM, Gaissert HA, Grillo HC, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg. 2005; 130:1413–1421.

10. Kim DJ, Yang WI, Choi SS, Kim KD, Chung KY. Prognostic and clinical relevance of the World Health Organization schema for the classification of thymic epithelial tumors: a clinicopathologic study of 108 patients and literature review. Chest. 2005; 127:755–761.

11. Ströbel P, Bauer A, Puppe B, Kraushaar T, Krein A, Toyka K, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol. 2004; 22:1501–1509.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download