Abstract
Intermittent pneumatic compression (IPC) device is an effective method to prevent deep vein thrombosis. This method has been known to be safe with very low rate of complications compared to medical thromboprophylaxis. Therefore, this modality has been used widely in patients who underwent a hip fracture surgery. We report a patient who developed extensive bullae, a potentially serious skin complication, beneath the leg sleeves during the use of IPC device after hip fracture surgery.
Intermittent pneumatic compression (IPC) device is an effective means to prevent deep vein thrombosis,1-4 and has been known to be safe with very low rate of complications compared to medical thromboprophylaxis.5 Therefore, this modality has been used widely in patients who underwent a hip fracture surgery.1-3,6
We report a patient who developed extensive bullae, a potentially serious skin complication, beneath the leg sleeves during the use of IPC device after hip fracture surgery.
An 81-year-old woman, who sustained an intertrochanteric fracture on her left hip, was admitted via emergency room. She had been medicated with a beta-blocker for the treatment of hypertension. Otherwise, she did not take any medicine including steroids or anti-platelet drugs. She had no history of skin allergies, and preoperative laboratory examination revealed no evidence of coagulopathy. She underwent a cementless bipolar hemiarthroplasty under a spinal anesthesia. No special event occurred during the operation.
Postoperatively, IPC device (Kendall SCD EXPRESS™; Covidien, Mansfield, MA, USA) was applied on her both legs. According to a manufacture's brochure, the sleeve is made of a soft, lightweight, non-woven material, which can promote patient's comfort and compliance, and the microprocessor controls a 11-second-compression cycle to ensure 45 mm Hg-pressure delivery at the ankle, with resulting pressures of 40 mm Hg and 30 mm Hg sequentially at the calf and thigh.
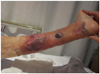
There were no special events during hip fracture surgery, and her vital signs were stable on immediate postoperative period. But the patient became delirious on postoperative day 1. On the postoperative day 3, when she recovered from the delirium, she complained of left leg pain, and extensive large bullous skin lesions were found in her left leg. The lesions developed circumferentially beneath the IPC sleeves (Fig. 1). On physical examination, distal pulses, sensation and motor function were intact.
Immediately after the detection of skin lesions, IPC device was removed. The patient was consulted to a dermatologist and a diagnosis of fragile skin was made. Hemorrhagic fluid was aspirated, and wet dressing was applied. After 7 days, the skin lesions improved and the hemorrhagic bullae disappeared.
Venous thromboembolism is a frequent and serious complication after hip fracture surgery in elderly patients. The IPC has been known to be effective in preventing venous thrombosis with far less morbidities than pharmacologic prophylaxis.5 Compartment syndrome due to malfunctioning sleeves or a prolonged use has been reported.7 However, no skin complication has been reported after the use of IPC so far.
Our report showed that a bullous skin complication can occur even with recommended pressurization of 40-45 mm Hg during the use of IPC when the patient's skin is fragile.
In our patient, the detection of the skin lesion was delayed by 2 or 3 days because the patient was delirious postoperatively. Delirium is commonly detected in elderly patients who underwent hip fracture surgery, that is characterized a change of mental status with attention, awareness deficits, and loss of cognitive and perceptive functions.8,9
When IPC device is applied on elderly patients with fragile skin and delirious condition, skin condition of the legs should be checked frequently during the application.
Figures and Tables
ACKNOWLEDGEMENTS
The authors did not receive any outside funding or grants in support of their research for or preparation of this work. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
1. Fordyce MJ, Ling RS. A venous foot pump reduces thrombosis after total hip replacement. J Bone Joint Surg Br. 1992. 74:45–49.
2. Pitto RP, Hamer H, Heiss-Dunlop W, Kuehle J. Mechanical prophylaxis of deep-vein thrombosis after total hip replacement a randomised clinical trial. J Bone Joint Surg Br. 2004. 86:639–642.
3. Warwick D. Intermittent pneumatic compression prophylaxis for proximal deep venous thrombosis after total hip replacement. J Bone Joint Surg Am. 1998. 80:141–142.
4. Warwick D, Harrison J, Glew D, Mitchelmore A, Peters TJ, Donovan J. Comparison of the use of a foot pump with the use of low-molecular-weight heparin for the prevention of deep-vein thrombosis after total hip replacement. A prospective, randomized trial. J Bone Joint Surg Am. 1998. 80:1158–1166.

5. Freedman KB, Brookenthal KR, Fitzgerald RH Jr, Williams S, Lonner JH. A meta-analysis of thromboembolic prophylaxis following elective total hip arthroplasty. J Bone Joint Surg Am. 2000. 82-A:929–938.

6. Handoll HH, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev. 2002. CD000305.

7. Werbel GB, Shybut GT. Acute compartment syndrome caused by a malfunctioning pneumatic-compression boot. A case report. J Bone Joint Surg Am. 1986. 68:1445–1446.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download