Abstract
A 31-year-old Korean male presented with altered consciousness and severe headache. Brain MRI delineated focal leptomeningeal enhancement without any intracerebral lesions. Diagnosis was made based on a brain biopsy showing anaplastic large cell lymphoma (ALCL), immunohistochemical stains revealing positivity for anaplastic lymphoma kinase (ALK) and an absence of involvement in any other organs; specifically, the primary central nervous system ALK+ALCL. Complete remission was achieved following 5 cycles of systemic chemotherapy with a high dose of Methotrexate and a simultaneous 7 cycles of intrathecal triple chemotherapy. Diagnosis of primary leptomeningeal ALK+ALCL is challenging given its rarity and non-specific symptoms along with non-pathognomonic radiologic findings. We present the first case of primary leptomeningeal ALK-positive ALCL where the clinical course, pathologic characteristics and treatment modality are described as well as a review of literature.
Anaplastic large cell lymphoma (ALCL), which had first been described in 1985, was acknowledged as a distinct clinicopathologic entity in 2001.1,2 The 4th edition of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues in 2008 states that anaplastic lymphoma kinase (ALK) positive ALCL [ALK(+)ALCL] must be distinguished from the provisional entity of ALK-negative ALCL [ALK(-)ALCL].3,4 Although ALCL is primarily a nodal disease, extranodal involvement is not uncommon.5,6 Moreover, ALK(+)ALCL was reported to have a higher frequency of extranodal involvement compared to ALK(-)ALCL; skin (21%) was the most frequently involved organ followed by bone (17%) and soft tissues (17%).7 Primary involvement in the central nervous system (CNS) of ALK(+)ALCL, however, is exceptional and only eight cases of primary CNS ALCL with ALK positivity have been reported so far to our knowledge.5,8-12 Although systemic ALK(+)ALCL tends to present as an aggressive stage III or IV disease, it has been known to be more responsive to chemotherapy than ALK(-)ALCL, which contributes to better prognosis of the former than the latter.2,5-7,13 Whereas the overall incidence of primary CNS lymphoma was estimated to be 0.47 per 100,000 person-years, only 14 cases of primary CNS ALCLs had been reported by 2007: seven ALK(+)ALCLs, four ALK(-)ALCLs and the remaining three ALCLs with ALK positivity untested.5,7,14 Seven (50%) of these 14 experienced a rapidly fatal course leading to death and the mortality of primary CNS ALCL was reported to be greater than that of other types of CNS lymphomas in general.5,8,9,15-17 Treatment of choice for primary CNS ALK(+)ALCL has not been established. In this report, we describe the first case of primary leptomeningeal ALK(+)ALCL of an adult man who was successfully managed with systemic chemotherapy along with intrathecal chemotherapy without radiotherapy.
A 31-year-old Korean male was brought to the emergency department (ED) due to altered consciousness. At the ED, he was drowsy and complained of a severe headache, weakness in his left arm and difficulty speaking. He stated that he had been otherwise healthy and no laboratory results suggested immunodeficiency including human immunodeficiency virus. Magnetic resonance imaging (MRI) of his brain delineated leptomeningeal enhancement in the right temporal and insular gyri (Fig. 1) and cerebrospinal fluid (CSF) analysis via lumbar puncture showed 90 WBC/mm3 with 67% of lymphocytes. Differential diagnosis at the time included viral meningoencephalitis and tuberculosis (TB) meningoencephalitis. Antiviral and anti-TB therapy were started, neither of which was effective. His headache and altered mental status were responsive only to Mannitol and Dexamethasone. A follow-up MRI and CSF analysis, performed 4 weeks after the initial visit to the ED, revealed more prominent enhancement in the affected gyri and higher WBC counts (150/mm3) with elevated CSF pressure (26.5 cm H2O), respectively. On the second CSF analysis via lumbar puncture, a few scattered atypical lymphoid cells were identified.
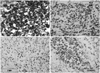
On the 39th hospital day, he underwent a brain biopsy following a craniotomy. The histologic section showed brain parenchyma infiltrated by numerous small-to-medium sized neoplastic cells (Fig. 2A). These neoplastic cells had irregular nuclei with a moderate amount of cytoplasm (Fig. 2B). Large atypical cells with horseshoe shaped nuclei, which are hall mark cells of anaplastic large cell lymphoma, were also present (Fig. 2B, inset). Immunohistochemical stains revealed immunopositivity for CD30, ALK, Granzyme B, CD45RO, CD5 and EMA (Fig. 3), but negative immunoreactivity for LCA, CD20, CD3, CD2, CD4, CD8, CD15, CD43, CD68, BCL-2, CD56 and TIA-1. ALK was positive in both the nucleus and cytoplasm. Fluorescence in situ hybridization (FISH) was undertaken using a break apart probe, which demonstrated translocations involving ALK gene (Fig. 4). The case was diagnosed with malignant lymphoma of T-cell lineage, ALK+ALCL.
CT scans of his abdomen and chest failed to show any lesion suggesting involvement of lymphoma. A bone marrow biopsy was performed, which was negative, and a test for the Epstein-Barr virus was negative as well. On the seventh post-biopsy day, he reported blindness in both eyes along with severe headache, which indicated most likely very high intracranial pressure (ICP). CSF pressure was measured to be over 40 cm H2O. Systemic high dose methotrexate (HD MTX, 8 g/mm2/day) and intrathecal (IT) triple chemotherapy (MTX, Ara-C and Hydrocortisone) were immediately started. After this, four more cycles of HD MTX were repeated with the interval of three weeks. Seven cycles of IT triple chemotherapy were simultaneously performed in total with the interval of 4 days.
Other symptoms and problems that occurred during the chemotherapy were generalized tonic clonic seizure, tremor in his jaw with dystonic feature, hypotension, pneumonia, central diabetes insipidus (DI) and minimal intracerebral hemorrhage (ICH) in the right temporal lobe accompanied with intraventricular hemorrhage (IVH). ICH and IVH did not require a surgical decompression. A series of follow-up MRI undertaken after the fifth HD-MTX revealed no residual enhancement, which suggested complete remission (CR). Although mild quadriparesis and jaw dystonia remained, no evidence of recurrence has been noted for more than six months since CR.
Diagnosis of primary CNS ALK+ALCL requires several critical steps including: clinical suspicion even when no gross mass exists, biopsy in a timely manner, a thorough immunohistochemical study, and exclusion of metastasis. Given that extranodal involvement of ALK(+)ALCL is relatively common, diagnosis of primary CNS ALK(+)ALCL must be made only after excluding other involvement.5,6 Primary CNS lymphomas with predominant involvement of leptomeninges are harder to diagnose because both aseptic and septic meningoencephalitis should be ruled out first. Our patient was assumed to have viral or mycobacterial meningoencephalitis given no gross mass seen upon brain MRI. Primary leptomeningeal lymphomas account for only about 7% of all adult primary CNS lymphomas.18 As seen in Table 1, two of eight cases harbored predominantly leptomeningeal involvement with no intracerebral lesions and neither of them was an adult.11 Our case is the first primary leptomeningeal ALCL with ALK positivity in an adult. It would inevitably take longer for patients with grossly sheer leptomeningeal involvement to undergo a biopsy than those who have one or more intracerebral lesions.
Among 8 reported cases, histologic subtypes were described in only 4 cases. Three of these 4 cases showed common histologic subtypes and the remaining 1 was a combined lymphohistiocytic and small cell variant. Our case was common histologic subtype. All reported cases were CD30 and ALK positive and have a T- or null cell phenotype, and most are EMA-positive. Our case was T-cell phenotype showing immunopositivity for CD45RO and CD5 but immunonegativity for CD3. As shown Figs. 3 and 4, ALK was positive in both the nucleus and cytoplasm. ALK gene translocation was also detected by FISH. These findings are consistent with the ALCL of NPM-ALK fusion gene, as a consequence of the t(2;5)(2p23;5q35). The majority (60-80%) of ALK(+)ALCL display a characteristic chromosomal translocation involving the NPM gene located at 5q35 with the ALK gene on 2p23.4
Treatment of choice for primary CNS ALK(+)ALCL has not been established mainly due to its great rarity. For pediatric patients, combined systemic chemotherapy and CNS radiation is usually administered, although a recent report suggested that combination of systemic and intrathecal chemotherapy without cranial radiotherapy should be safer and more efficient.19,20 The risk of whole-brain irradiation leading to long-term complications is known to be substantial in children; therefore, sooner consensus on the most effective chemotherapeutic regimen should be reached in order to avoid or at least delay radiotherapy.21 Our patient achieved CR following 5 cycles of HD MTX along with 7 cycles of IT triple chemotherapy without craniospinal radiotherapy. He suffered from several temporary problems such as severe headache, blindness, seizure and central DI. Long-term complications included quadriparesis and dystonia in the jaw. Some of these temporary and permanent complications were assumed to derive from severely elevated intracranial pressure, rather than from lymphoma itself. Therefore, these complications may have been avoided if the chemotherapy had been started sooner enough. As mentioned earlier, grossly sheer leptomeningeal involvement without an intracranial lesion contributed to belated brain biopsy leading to delayed commencement of chemotherapy. According to a report in 2007, seven of 14 patients with primary CNS ALCL died; two of them had ALK(+)ALCL, three had ALK(-)ALCL and the remaining two did not go through the test for ALK positivity.5 Given the insufficient sample size to be statistically significant, prognosis of primary CNS ALK(+)ALCL compared to ALK(-)ALCL is inconclusive.5,9,11 In addition, there are no statistically significant data on prognosis of primary CNS ALCL compared to CNS lymphomas in general, but the mortality of the former seemed to be greater than that of the latter.5,17 Systemic ALK(+)ALCL has been known to be more responsive to chemotherapy leading to better prognosis than ALK(-)ALCL, but this does not seem to apply to primary CNS ALCLs.5
In conclusion, this is the first reported case of primary leptomeningeal ALK(+)ALCL in an adult to our knowledge. Early detection is of vital importance in terms of both mortality and morbidity. Physicians should consider the possibility of leptomeningeal lymphomas in a patient with symptoms mimicking those of meningitis including increased ICP with meningeal irritation, CSF analysis revealing atypical lymphoid cells, no gross mass detected in the brain MRI and lack of symptomatic improvement by antiviral, antibiotic or anti-TB medications.
Figures and Tables
Fig. 1
Magnetic resonance imaging of his brain showed hyperintense signal in the right temporal sulci from the Flair image (A) and leptomeningeal enhancement in the right temporal and insular gyri from Gadolinium enhanced image (B).
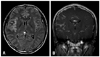
Fig. 2
The histologic section showed brain parenchyma infiltrated by numerous small-to-medium sized neoplastic cells with perivascular cuffing of neoplastic cells (A, HE ×100) and the neoplastic cells had irregular nuclei with a moderate amount of cytoplasm (B, HE ×400). Large atypical cells with horseshoe shaped nuclei, which are hallmark cells of anaplastic large cell lymphoma, were also present (B, inset, HE ×1000)

Fig. 3
Immunohistochemical stains revealed immunopositivity for CD30, ALK, Granzyme B, CD45RO and EMA. ALK, anaplastic lymphoma kinase.
References
1. Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985. 66:848–858.
2. Jaffe ES, Harris NL, Stein H, Wardiman JW. Tumors of haematopoietic and lymphoid tissues. 2001. Lyon: IARC Press.
3. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011. 117:5019–5032.

4. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2008. 4th ed. Lyon, France: IARC Press.
5. Karikari IO, Thomas KK, Lagoo A, Cummings TJ, George TM. Primary cerebral ALK-1-positive anaplastic large cell lymphoma in a child. Case report and literature review. Pediatr Neurosurg. 2007. 43:516–521.

6. Penny RJ, Blaustein JC, Longtine JA, Pinkus GS. Ki-1-positive large cell lymphomas, a heterogenous group of neoplasms. Morphologic, immunophenotypic, genotypic, and clinical features of 24 cases. Cancer. 1991. 68:362–373.

7. Falini B, Pileri S, Zinzani PL, Carbone A, Zagonel V, Wolf-Peeters C, et al. ALK+ lymphoma: clinico-pathological findings and outcome. Blood. 1999. 93:2697–2706.
8. Abdulkader I, Cameselle-Teijeiro J, Fraga M, Rodriguez-Núnez A, Allut AG, Forteza J. Primary anaplastic large cell lymphoma of the central nervous system. Hum Pathol. 1999. 30:978–981.

9. George DH, Scheithauer BW, Aker FV, Kurtin PJ, Burger PC, Cameselle-Teijeiro J, et al. Primary anaplastic large cell lymphoma of the central nervous system: prognostic effect of ALK-1 expression. Am J Surg Pathol. 2003. 27:487–493.
10. Ponzoni M, Terreni MR, Ciceri F, Ferreri AJ, Gerevini S, Anzalone N, et al. Primary brain CD30+ ALK1+ anaplastic large cell lymphoma ('ALKoma'): the first case with a combination of 'not common' variants. Ann Oncol. 2002. 13:1827–1832.

11. Merlin E, Chabrier S, Verkarre V, Cramer E, Delabesse E, Stéphan JL. Primary leptomeningeal ALK+ lymphoma in a 13-year-old child. J Pediatr Hematol Oncol. 2008. 30:963–967.

12. Ozkaynak MF. Favorable outcome of primary CNS anaplastic large cell lymphoma in an immunocompetent patient. J Pediatr Hematol Oncol. 2009. 31:128–130.

13. Shiota M, Nakamura S, Ichinohasama R, Abe M, Akagi T, Takeshita M, et al. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood. 1995. 86:1954–1960.

14. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer. 2011. 105:1414–1418.

15. Buxton N, Punt J, Hewitt M. Primary Ki-1-positive T-cell lymphoma of the brain in a child. Pediatr Neurosurg. 1998. 29:250–252.

16. Goldbrunner R, Warmuth-Metz M, Tonn JC, Vince GH, Roosen K. Primary Ki-1-positive T-cell lymphoma of the brain--an aggressive subtype of lymphoma: case report and review of the literature. Surg Neurol. 1996. 46:37–41.

17. Paulus W, Ott MM, Strik H, Keil V, Müller-Hermelink HK. Large cell anaplastic (KI-1) brain lymphoma of T-cell genotype. Hum Pathol. 1994. 25:1253–1256.

18. Lachance DH, O'Neill BP, Macdonald DR, Jaeckle KA, Witzig TE, Li CY, et al. Primary leptomeningeal lymphoma: report of 9 cases, diagnosis with immunocytochemical analysis, and review of the literature. Neurology. 1991. 41:95–100.

19. Taga T, Sakaue Y, Anzai Y, Takeuchi Y, Ohta S. Pediatric primary leptomeningeal lymphoma treated without cranial radiotherapy. Pediatr Blood Cancer. 2007. 48:477–478.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download