Abstract
Purpose
It is still unclear whether the longitudinal anal muscles or conjoint longitudinal coats (CLCs) are attached to the vagina, although such an attachment, if present, would appear to make an important contribution to the integrated supportive system of the female pelvic floor.
Materials and Methods
Using immunohistochemistry for smooth muscle actin, we examined semiserial frontal sections of 1) eleven female late-stage fetuses at 28-37 weeks of gestation, 2) two female middle-stage fetus (2 specimens at 13 weeks), and, 3) six male fetuses at 12 and 37 weeks as a comparison of the morphology.
Results
In late-stage female fetuses, the CLCs consistently (11/11) extended into the subcutaneous tissue along the vaginal vestibule on the anterior side of the external anal sphincter. Lateral to the CLCs, the external anal sphincter also extended anteriorly toward the vaginal side walls. The anterior part of the CLCs originated from the perimysium of the levator ani muscle without any contribution of the rectal longitudinal muscle layer. However, in 2 female middle-stage fetuses, smooth muscles along the vestibulum extended superiorly toward the levetor ani sling. In male fetuses, the CLCs were separated from another subcutaneous smooth muscle along the scrotal raphe (posterior parts of the dartos layer) by fatty tissue.
The smooth muscle sheet running along the anal canal, interposing between the external and internal anal sphincters (EAS, IAS), has been termed the longitudinal anal muscles1,2 or the conjoint longitudinal coats (CLCs).3 Macchi, et al.1 reviewed previous research of this structure, focusing especially on differences among previous reports, and measured the thickness of the CLCs in 16 cadavers of elderly individuals. To verify the origin of the term "conjoint", our group has described in detail the morphology at the junction between the CLCs and the levator ani muscle.4 We found that the CLCs receive smooth muscles from 1) the rectal longitudinal muscle layer and 2) the intramuscular connective tissue or perimysium of the levator ani muscle. The levator ani muscle, the strongest striated muscle in the pelvic floor, is associated with abundant smooth muscle as a covering fascia and perimysium in both males and females.5,6 However, among the connective tissue-like smooth muscles, only the male rectourethralis muscle is probably the best known.7,8 Using immunohistochemistry, Matsubara, et al.9 and Uchimoto, et al.10 demonstrated the rectourethralis muscle connecting with the fascia and perimysium of the levator ani.
Macchi, et al.1 and Shafik2 reported that, at the anterior part of the CLCs, multiple fascial septa are predominantly located at the distal end and penetrate the EAS, reaching the deep aspect of the dermis. Shafik emphasized that the septa run downward, decussate with contralateral ones, and form the central tendon. Likewise, according to Lesshaft,1 the external fibers of the CLCs insert into the superior fascia of the pelvic diaphragm (i.e., a fascia of the levator ani) and the internal fibers run into the EAS and the deep part of the anal skin. However, those studies did not seem to address the issue of whether the smooth muscles were attached to the vagina. To our knowledge, no previous report has described the morphology of the female CLCs at or near the vaginal attachment of the levator ani. Knowledge of the topographical relationship between the CLCs and the vagina seems to be critical for evaluating the hypothetical integrated supportive system of the female pelvic floor.11 Consequently, using immunohistochemistry for alpha smooth muscle actin, we histologically examined female late-stage fetuses in which the CLCs were likely to have fully developed without degeneration, and we compared the findings with those in male fetuses as well as female fetuses at earlier stages.
This study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We examined paraffin-embedded tissues or sections of 13 female and 6 male fetuses: 1) eleven female late-stage fetuses [28-37 weeks of gestation; crown-rump length (CRL), 220-320 mm]; 2) two female middle-stage fetuses (2 specimens at 13 weeks, CRL 90 and 100 mm) and, as a comparison of the morphology, 3) four male late-stage fetuses (all 37 weeks, CRL 280-300 mm) and 4) two male middle-stage fetuses (all 12 weeks, CRL 60-70 mm). All of the specimens were in the possession of the Embryology Institute of the Universidad Complutense, Madrid, being the products of urgent abortion, miscarriages or ectopic pregnancies managed at the Department of Obstetrics of the University. The donated fetuses had been fixed in 10% w/w formalin solution and stocked in the same solution for 3-12 months. After trimming of the tissue mass including the pelvic floor, the specimens were decalcified in EDTA solution at 4℃ for 3 days (0.5 mol/L, pH 7.5; Decalcifying solution B, Wako, Tokyo, Japan). Approval for the study was granted by the ethics committee of the university.
After routine procedures for paraffin-embedded histology, sections were cut frontally at a thickness of 5 µm, at intervals of 0.5 mm (late-stage fetuses) or 20 µm (middle-stage fetuses). There were around 50 sections for each of the late-stage fetuses and around 200 for the early stage. Most sections were stained with hematoxylin and eosin (HE), while some (10-20 sections per specimen) were subjected to immunohistochemical staining. The primary antibody used were 1) rabbit polyclonal anti-human alpha smooth muscle actin (dilution, 1 : 100; 5 µg/mL; Dako Cytomation, Kyoto, Japan) and 2) mouse monoclonal anti-human striated muscle myosin heavy chain (dilution 1 : 100, 5 µg/mL; Dako Cytomation, Kyoto, Japan). The sections challenged by the first antibody were incubated overnight at 4℃. Pretreatment in an autoclave was not conducted because of the fragile nature of the fetal tissues. After that, the sections were washed and then incubated with horseradish peroxidase (HRP), and antigen-antibody reactions were detected by the HRP-catalyzed reaction with diaminobenzidine (Dako Chem Mate Envison Kit; Dako, Carpinteria, CA, USA). Weak counterstaining with hematoxylin was also performed. The endothelium of the artery and vein as well as any smooth muscle is positive in the immunohistochemistry.12,13 Staining images were compared with the negative control, and the expression of protein was confirmed.
The antibody for striated muscles was used only for two female middle-stage fetuses at 13 weeks. In order to correlate HE staining with smooth muscle immunohistochemistry and to reduce the gap of information obtained with these two methods, higher-magnification views of HE sections corresponding to a part of low-magnification views of immunohistochemistry will be shown for the late-stage specimens.
At 13 weeks, smooth muscles were seen along the vestibulum, urethra and rectum (Fig. 1). The longitudinal and circular smooth muscle layers are discriminated in the rectal wall. The vagina did not accompany smooth muscle coat except for the inferior end (Fig. 1C). The CLCs were not evident in the middle-stage specimens, although the levator ani and EAS were already well developed. However, at 13 weeks, inferior parts of smooth muscles coat of the vestibule extended superiorly toward the inferior edge of the levator ani muscle slings (Fig. 1A and B). Likewise, the rectal longitudinal layer issued a few smooth muscle superiorly (Fig. 1C). The bulbospongiosus muscle was located closely to the EAS and both muscles were difficult to discriminate even using immunohistochemsitry of striated muscle myosin (Fig. 1E and F). The vestibular bulb was not evident between the EAS and bulbospongiosus.
In the female fetuses at the late stage, the CLCs consistently (11/11) extended into the subcutaneous tissue along the vaginal vestibule anterior to the external anal sphincter (Figs. 2 and 3). These subcutaneous smooth muscles were separated from the vaginal smooth muscle layer by a vaginal wall composed of connective tissue (0.5-1.0 mm in thickness) containing abundant veins (e.g., Fig. 3B). In anterior sections including the vaginal wall (Figs. 2A and B, 3A and B), part of the CLCs inserted into or attached to the IAS and EAS, but the major part extended into the subcutaneous tissue. However, in posterior sections in which the IAS was clearly identifiable (Figs. 2C and 3C), the CLCs became restricted to a narrow space between the IAS and EAS. The anterior part of the CLCs (or, simply, the anterior CLCs) originated from the perimysium of the levator ani muscle without any contribution of the rectal longitudinal muscle layer.
In the posterior site, the rectal longitudinal muscle joined the longitudinal anal muscles in 6 of 11 late-stage specimens (Fig. 3C). In the late-stage specimens, thick bundles or a mass of smooth muscle were seen in two larger specimens (37 weeks) immediately anterior to the anal canal (Fig. 3). This bulky smooth muscle was irregularly arrayed and was not in contact with the anal longitudinal muscle layer. No bundle of the CLCs penetrated the EAS (Figs. 2C and 3C), but in more posteriorly, such penetrating smooth muscle bundles appeared and divided the EAS into several masses. The CLCs inferior bundles dividing the EAS were consistently seen in all 11 specimens. In the posterior area, abundant smooth muscle fibers of the CLCs also inserted into the IAS: more strictly, they connected with the type VI collagen-made epimysium of the IAS muscle fibers (Figs. 2C and 3C). We did not find any direct connection between the smooth muscle fibers of the CLCs and the IAS.
Lateral to the anterior CLCs, the external anal sphincter also extended anteriorly toward the vaginal side walls in late-stage specimens. The bulbospongiosus muscle attached to the EAS, but this attachment is not shown in the late-stage (Figs. 2 and 3) but in the middle-stage (Fig. 1). The bulbospongiosus and vestibular bulb were located anterosuperiorly to the anterior CLCs. The perineal membrane was not evident in all fetuses, but the urethral striated sphincter (urethral rhabdosphincter) inserted into a tight space between the vestibular bulb and vaginal wall in 3 of the 11 late-stage specimens.
In two middle-stage fetuses, the bulbospongiosus muscle was located closely to the EAS and anal canal (Fig. 4). A smooth muscle mass was seen developing between the rectum and levator ani (Fig. 4F and G). This smooth muscle mass was continuous with another mass, the rectourethralis muscle, in the anterior side of Fig. 4. However, the connective tissue-like smooth muscles were restricted at and near the inferomedial edge of the levator ani but they did not extend inferiorly toward the subcutaneous tissue (Fig. 4). The definite CLCs were not seen in the male middle stage. In 4 late-stage fetuses, subcutaneous smooth muscles were always evident along the midline raphe-like skin ligament (Fig. 5A) and were continuous with the dartos layer (tunica dartos) anteriorly. The raphe-like structure was, on the superior side, continuous with the midline septum of the bulbus penis or bulbospongiosus. In all 4 specimens, the subcutaneous smooth muscle along the raphe-like structure or bulbospongiosus was separated from the anterior wall of the anal canal by the fatty tissue. Thus, we found no sections that included both the raphe-like structure and CLCs. The CLCs were composed of tightly packed smooth muscle fibers, in contrast to female ones in which loose tissue lay between smooth muscle fibers. The thick CLC layer penetrated the EAS and divided it into 4-5 muscle bundles (Fig. 5B), but the CLCs did not extend anteriorly. The longitudinal muscle layer of the rectum joined the CLCs in all 4 late-stage specimens. Insertions of the CLCs into the IAS appeared to be much fewer in number per section than in females, but this was an artifact due to the tightly packed CLCs running toward the EAS.
The present study demonstrated that, the anterior part of the CLCs (the anterior CLCs) in females, extends into the subcutaneous tissue along the vagina and vaginal vestibule. The anterior CLCs were not located between the EAS and IAS, but originated from muscle-associated connective tissues of the levator ani and ended at the subcutaneous tissue. The present anterior CLCs were located much more anteriorly than the "anterior part of the longitudinal anal muscles" described by Macchi, et al.1 and Shafik2 because the latter penetrate the EAS. According to observations of 2 middle-stage fetuses, most parts of the female CLCs seemed to originate from the smooth muscle coat of the vestibulum, not from the rectum. Thus, the function also seems to be different from the proper or posterior part of CLCs that cooperate during contractions of the levator ani, EAS and IAS (for details, see below). Notably, such an anterior extension was not seen in male fetuses. Moreover, the male CLCs were composed of tightly packed smooth muscle fibers, in contrast to the female anterior CLCs with abundant loose tissues between muscle fibers. On the basis of topographical anatomy, the female anterior CLCs seem to correspond to a bulky subcutaneous smooth muscle mass that Soga, et al.14 described in elderly women as the "lateral extension of the perineal body", supporting the vaginal vestibule by transmission of force from the EAS and levator ani. In contrast, the perineal membrane, which seems to develop later than the CLCs, is most likely located in the deep or anterosuperior side of the subcutaneous anterior CLCs according to the topographical relationship between the CLCs and the vestibular bulb. To summarize the topographical anatomy, we try to interpose our "fetal" observations into a schematic representation based on the "adult" anatomy (Fig. 6).
According to DeLancey,15 the perineal body and perineal membrane together play a critical role in level III support of the female pelvic floor, although the perineal membrane which he described may differ from the actual morphology described by Kato, et al.16 The level II supportive tissues are continuous with the level III support, i.e. the fascial connection between the levator ani and the vagina. Such a connection would be provided by the present anterior CLCs. In fact, Soga, et al.14 considered that the lateral extension of the perineal body was most likely to correspond to DeLancy's perineal membrane in terms of topographical anatomy. The lateral extension of the perineal body reaches 20 mm in width in multiparous women and provides major anterior insertions for the EAS.14 Thus, the EAS as well as the CLCs seem to play a dynamic role in supporting the rectal-vaginal interface. Herein, the term "dynamic" is based on the smooth muscle physiology that contrasts with the static support provided by type I collagen fibers such as normal tendons and ligaments.
The "integrated pelvic floor theory"11 attributed a key role to the CLCs in the dynamics and statics of the pelvic viscera, being involved in the opening and closure of the urethra and anorectal canal. According to Petros,11 the CLCs, with their vertical course, create a downward force for bladder neck closure during effort and stretch the outflow tract open during micturition. However, the CLCs were located distantly from the female urethra or its sphincters, and ended more posteriorly around the vagina and vaginal vestibule. Parts of the urethral striated sphincter (the urethrovaginal sphincter) extend between the bulbopongiousus muscle and the vagina (i.e., the same layer as the perineal membrane16), whereas the CLCs are located inferoposteriorly or superficially to the bulbospongiosus (Fig. 6).
In contrast to the urethra, the anal and perineal skin seems to be elevated by the CLCs. In fact, Shafik2 called the CLCs the "elevator ani muscle". He also considered that, during their elevation, the CLCs angulate the tip of the levator ani downwards, creating the anorectal angle. However, this hypothesis may be at odds with the aforementioned elevation of the skin: the skin is too fragile to fix the levator ani and anal canal. On the basis of a detailed immunohistochemical study of adult specimens, Arakawa, et al.4 hypothesized that the CLCs conduct a force generated by the levator ani contraction to the IAS and EAS with a modification and time-lag. Because the CLCs are smooth muscles, this transmission would be different from that of a type-1 collagen-made tendon. Smooth muscle is more resistant to tension than elastic fibers. In comparison with static collagenous and elastic fibers, smooth muscle seems to resist stretch stress due to its dynamic biomechanical characteristics. Extracellular collagenous and elastic fibers would be damaged by excessive tension, whereas smooth muscle can become highly relaxed under various humoral and nervous influences. Consequently, the female anterior CLCs seem to transmit the levator ani contraction to the vaginal side-wall through a modification of smooth muscle physiological characteristics in order to avoid vaginal prolapse rather than anal prolapse.
In addition, the present study demonstrated a close topographical relationship between the EAS and the bulbospongiosus. This seems to be consistent with a report by van der Putte,17 who hypothesized that the bulbospongiosus and the deep portion of the EAS have a common anlage. He also described details of the perineal raphe, but strangely did not describe the subcutaneous smooth muscle that we observed along the male raphe. The morphology of the perineal raphe is a critical feature for discrimination of anorectal malformations.18 However, to our knowledge, there has been no description of the subcutaneous smooth muscle including the CLCs in pathological conditions involving the raphe.17,19,20 Because gender differences were clearly evident in the anterior CLCs, anomalies involving the vagina would seem to accompany abnormalities of the CLCs, including their absence. Further studies are also required using young adult specimens because our previous studies on the longitudinal smooth muscles4,14 were based on histology of elderly cadavers.
Figures and Tables
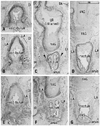 | Fig. 1Developing vaginal vestibulum accompanies smooth muscle coat at 13 weeks. Frontal sections. Panels A-D are immunohistochemistry for smooth muscle actin while panels E-G are for striated muscle myosin. Panels B and E (C and F, D and G) show the near sections. Panel A (or panel D) is the most anterior side (or posterior side) in the figure. Intervals between panels are 0.5 mm (A-B, B-C) and 0.6 mm (C-D), respectively. The developing vestibulum accopanies a smooth muscle cost and it issues muscle fibers superiorly (arrows in panels A and B). Likewise, a few smooth muscles extend from the rectum (arrowheads in panel C). The urethra (UR) or urogenital sinus is dilated and surrounded by smooth muscle layer (panel C). In contrast, most or upper parts of the vagina do not accompany smooth muscles (panel D). The striated muscle immunohistochemistry (panels E and F) displays a close relation between the bulbospongiosus (BS) and the external anal sphincter (EAS). IC, ischiocavernosus muscle; IR, ischial ramus of the pelvis; LA, levator ani; OI, obturator internus muscle; VAG, vagina. |
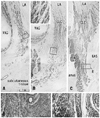 | Fig. 2Longitudinal anal muscles in a female 28-week fetus. Frontal sections. Smooth muscle actin immunohistochemistry and HE staining. Panel A (or Panel C) is the most anterior (or posterior) in the figure. Intervals between panels are 3.0 mm (A-B) and 4.5 mm (B-C), respectively. Panel D (or Panel E) is a higher magnification view of a square in panel A (or panel C). An insert at the top of panel B is also a higher magnification view corresponding to a square in the panel. The conjoint longitudinal coat (CLC) originates from the perimysium of the levator ani (LA) and insert into the subcutaneous tissue (panels A and B) or on the external and internal anal sphincters (EAS, IAS; panel C). Asterisks in panel B indicate the most anterior part of the longitudinal muscle layer of the anal canal. VAG, the most posterior part of the vagina. In HE staining (panels D and E, insert of panel B), each fiber of smooth muscle bundles (i.e., CLC or IAS) carries an irregular surface similar to a feather in contrast to a smooth surface of the striated muscle (i.e., EAS). HE, hematoxylin and eosin; VAG, vagina. |
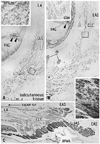 | Fig. 3Longitudinal anal muscles in a female 37-week fetus. Frontal sections. Smooth muscle actin immunohistochemistry and HE staining. Panel A (or Panel C) is the most anterior (or posterior) in the figure. The supero-inferior axis of panel C is different from panels A and B. Intervals between panels are 4.0 mm (A-B) and 6.0 mm (B-C), respectively. Inserts at the top of panels A and B are a higher magnification view corresponding to a square in the panel A or B, respectively. Another insert between panels B and C displays a square in panel C. The morphology of the conjoint longitudinal coat (CLC) is similar to that shown in Fig. 3. However, they provided thick bundles or a mass (star in panel B) in the immediately anterior side of the longitudinal muscle layer (asterisk in panel B) of the anal canal. Arrowheads indicate smooth muscles in the folds of the vagina. In HE staining (an insert for panel C), each fiber of smooth muscle bundles carries an irregular surface in contrast to a smooth surface of the striated muscle. EAS, external anal sphincter; IAS, internal anal sphincter; LA, levator ani; VAG, vagina; HE, hematoxylin and eosin. |
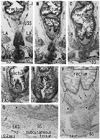 | Fig. 4Smooth muscle distribution along the male rectum at 12 weeks. Frontal sections. HE staining (panels A-E) and immunohistochemistry for alpha smooth muscle actin (panels F and G). Panel A (or Panel E) is the most anterior (or posterior) side in the figure. Intervals between panels are 0.2 mm (A-B), 0.3 mm (B-C), 0.4 mm (C-D) and 0.6 mm (D-E), respectively. Panel F (or G) is a higher magnification view of the lower part of panel C (or a square in panel D). Panel A includes the posterior margin of the urethra and urethral striated sphincter (UR&USS). The bulbospongiosus (BS) becomes smaller in the more posterior section (from panel A to D) and, in panels D and G, it shows the close relation with the external anal sphincter (EAS). Panels F and G display a smooth muscle mass (stars) at the inferomedial edge of the levator ani (LA) muscle, but the smooth muscles do not extend into the subcutaneous tissue. Asterisks in panel G indicate the smooth muscle layer in the rectal wall. The endothelium of the artery and vein (arrows in panels F and G) is also positive in the immunohistochemistry. bulbus, bulbus penis; P, pubis; IC, ischiocavernosus muscle; HE, hematoxylin and eosin. |
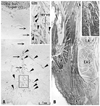 | Fig. 5Longitudinal anal muscles in a male 37-week fetus. Frontal sections. Smooth muscle actin immunohistochemistry and HE staining. Panel A, including the most posterior part of the scrotal raphe, is 5 mm anterior to panel B showing the anterolateral wall of the anus. Inserts at the top of panels A and B are a higher magnification view corresponding to a square in the panel A or B, respectively. Panel A displays subcutaneous smooth muscles (arrowheads) along and near the scrotal raphe (arrows). These smooth muscles are separated from the conjoint longitudinal coat (CLC in panel B) by the fatty tissue. The longitudinal anal muscles insert into the external anal sphincter (EAS), not into the internal sphincter (IAS). Asterisk in panel B indicates the longitudinal muscle layer of the rectum. A dark color in parts of the EAS is due to non-specific staining in immunohistochemsitry. LA, levator ani. |
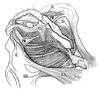 | Fig. 6A schematic representation of the female longitudinal anal muscles extending toward the vaginal vestibule. Posterosuperior view after complete dissection of the ischiorectal fossa. The present observations of the fetal conjoint longitudinal coat (CLC) are interposed onto the adult morphology. The CLC originates from the muscle associated connective tissues of the levator ani (LA) and extends antero-inferiorly into the subcutaneous tissue along and around the vaginal vestibule. These smooth muscles cross in the superficial or inferior side of the bulbospongiosus (BS). In accordance with the CLC, the external anal sphincter (EAS) also reaches the anterior site near the vaginal side walls, but we are unable to draw the morphology because of limitation of drawing skills. CO, coccygeus muscle; IC, ischiocavernosus muscle; OI, obturator internus muscle; PM, perineal membrane; SSL, sacrospinal ligament; STL, sacrotuberous ligament. |
References
1. Macchi V, Porzionato A, Stecco C, Vigato E, Parenti A, De Caro R. Histo-topographic study of the longitudinal anal muscle. Clin Anat. 2008. 21:447–452.

2. Shafik A. New concept of the anatomy of the anal sphincter mechanism and the physiology of defecation. II. Anatomy of the levator ani muscle with special reference to puborectalis. Invest Urol. 1975. 13:175–182.
3. Borley NR. Standring S, editor. Anal canal. Gray's Anatomy. 2008. 40th ed. London: Elsevier Churchill Linvingstone;1155–1160.
4. Arakawa T, Murakami G, Nakajima F, Matsubara A, Ohtsuka A, Goto T, et al. Morphologies of the interfaces between the levator ani muscle and pelvic viscera, with special reference to muscle insertion into the anorectum in elderly Japanese. Anat Sci Int. 2004. 79:72–81.

5. Murakami G, Nakajima F, Sato TJ, Tsugane MH, Taguchi K, Tsukamoto T. Individual variations in aging of the male urethral rhabdosphincter in Japanese. Clin Anat. 2002. 15:241–252.

6. Hirata E, Fujiwara H, Hayashi S, Ohtsuka A, Abe S, Murakami G, et al. Intergender differences in histological architecture of the fascia pelvis parietalis: a cadaveric study. Clin Anat. 2011. 24:469–477.

7. Brooks JD, Eggener SE, Chao WM. Anatomy of the rectourethralis muscle. Eur Urol. 2002. 41:94–100.

8. Porzionato A, Macchi V, Gardi M, Parenti A, De Caro R. Histotopographic study of the rectourethralis muscle. Clin Anat. 2005. 18:510–517.

9. Matsubara A, Murakami G, Arakawa T, Yasumoto H, Mutaguchi K, Akita K, et al. Topographic anatomy of the male perineal structures with special reference to perineal approaches for radical prostatectomy. Int J Urol. 2003. 10:141–148.

10. Uchimoto K, Murakami G, Kinugasa Y, Arakawa T, Matsubara A, Nakajima Y. Rectourethralis muscle and pitfalls of anterior perineal dissection in abdominoperineal resection and intersphincteric resection for rectal cancer. Anat Sci Int. 2007. 82:8–15.

11. Petros P. The female pelvic floor. 2004. Heidelberg: Springer.
12. Hayashi S, Murakami G, Ohtsuka A, Itoh M, Nakano T, Fukuzawa Y. Connective tissue configuration in the human liver hilar region with special reference to the liver capsule and vascular sheath. J Hepatobiliary Pancreat Surg. 2008. 15:640–647.

13. Miyake N, Hayashi S, Kawase T, Cho BH, Murakami G, Fujimiya M, et al. Fetal anatomy of the human carotid sheath and structures in and around it. Anat Rec (Hoboken). 2010. 293:438–445.

14. Soga H, Nagata I, Murakami G, Yajima T, Takenaka A, Fujisawa M, et al. A histotopographic study of the perineal body in elderly women: the surgical applicability of novel histological findings. Int Urogynecol J Pelvic Floor Dysfunct. 2007. 18:1423–1430.

15. DeLancey JO. Structural anatomy of the posterior pelvic compartment as it relates to rectocele. Am J Obstet Gynecol. 1999. 180:815–823.

16. Kato M, Matsubara A, Murakami G, Abe S, Ide Y, Sato I, et al. Female perineal membrane: a study using pelvic floor semiserial sections from elderly nulliparous and multiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2008. 19:1663–1670.

17. van der Putte SC. The devlopment of the perineum in the human. A comprehensive histological study with a special reference to the role of the stromal components. Adv Anat Embryol Cell Biol. 2005. 177:1–131.
18. Murphy F, Puri P, Hutson JM, Holschneider AM. Holschneider AM, Hutson JM, editors. Incidence and frequency of different types, and classification of anorectal malformations. Anorectal Malformations in Children. 2006. 40th ed. Berlin Heidelberg: Springer;163–184.

19. Stephens FD. Holschneider AM, Hutson JM, editors. Photographic album of anorectal malformations and the sphincter muscles. Anorectal Malformations in Children. 2006. 40th ed. Berlin Heidelberg: Springer;87–142.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download