Abstract
Purpose
This study aims to investigate the most appropriate effect-site concentration of remifentanil to minimize cardiovascular changes during inhalation of high concentration desflurane.
Materials and Methods
Sixty-nine American Society of Anesthesiologists physical status class I patients aged 20-65 years were randomly allocated into one of three groups. Anesthesia was induced with etomidate and rocuronium. Remifentanil was infused at effect-site concentrations of 2, 4 and 6 ng/mL in groups R2, R4 and R6, respectively. After target concentrations of remifentanil were reached, desflurane was inhaled to maintain the end-tidal concentration of 1.7 minimum alveolar concentrations for 5 minutes (over-pressure paradigm). The systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), heart rate (HR) and end-tidal concentration of desflurane were measured for 5 minutes.
Results
The end-tidal concentration of desflurane increased similarly in all groups. The SBP, DBP, MAP and HR within group R4 were not significantly different as compared with baseline values. However, measured parameters within group R2 increased significantly 1-3 minutes after desflurane inhalation. The MAP within group R6 decreased significantly at 1, 2, 4, and 5 minutes (p<0.05). There were significant differences in SBP, DBP, MAP and HR among the three groups 1-3 minutes after inhalation (p<0.05). The incidence of side effects such as hyper- or hypo-tension, and tachy- or brady-cardia in group R4 was 4.8% compared with 21.8% in group R2 and 15.0% in group R6.
Desflurane has the lowest solubility among volatile anesthetics; therefore, it allows for rapid induction and recovery from anesthesia, in addition to more precise adjustments of its concentration in response to surgical stimuli during anesthetic maintenance.1-5 This agent strongly resists biodegradation in the body and is devoid of potential toxicity in the tissue.3,6 However, an abrupt rise in the concentration of desflurane can lead to increases of blood pressure (BP) and heart rate (HR) following sympathetic hyperactivation.7
Such hemodynamic instability, though transient, may cause myocardial ischemia or cerebral hemorrhage in patients with cardiac or cerebrovascular disease. To blunt cardiovascular stimulation following a rapid increase of inspired desflurane concentrations, opioids (such as fentanyl or alfentanil) as well as esmolol or clonidine can be administered.8,9
Remifentanil, a recently developed opioid derivative, has a rapid onset with a blood-brain equilibrium half-life of 1-2 minutes. It is rapidly eliminated from the body by nonspecific esterase within the blood and tissues. This agent has a short context-sensitive half-time of 3 minutes regardless of infusion time, and does not accumulate even in patients with hepatic or renal failure.10,11 Such merits make remifentanil an appropriate pharmacologic agent for out-patient procedures or prolonged operations while maintaining the advantage of desflurane.12,13
An intravenous bolus of alfentanil effectively blunts the hemodynamic effect of desflurane.9 Compared with esmolol and clonidine, fentanyl may be the most clinically useful because it blunts the increase in HR and BP, has minimal cardiovascular depressant effects, and imposes little postanesthetic sedation.8 However, there has been no research on the effect-site concentration of remifentanil limiting cardiovascular instability. Therefore, we conducted a prospective study to determine the effect-site concentrations of remifentanil to prevent cardiovascular instability, which can develop during induction with high desflurane concentrations.
After approval by the Institutional Review Board and Ethics Committee (SNUMC/SNUHIRB of Seoul National University Hospital, Seoul, Korea, H-0904-016-276, chairman Prof BJ Park, 23 May 2009) and receiving individual written informed consent, 69 patients with the American Society of Anesthesiologists (ASA) physical status I between the age 20-65 years undergoing general anesthesia during the period of May 2009 to July 2009 were enrolled in this study. Patients were excluded if they met any of the following criteria: 1) habitual drug abuse of a sedative or hypnotic; 2) anemia (hematocrit less than 24%) or patients who require premedication; 3) high gastric content aspiration risk; 4) difficult airway; 5) patients with HR greater than 100 or less than 45 beats per minute before induction; 6) patients with the systolic blood pressure (SBP) greater than 160 mm Hg or less than 90 mm Hg before induction. Patients were randomly allocated into three groups by a computer-generated randomization schedule according to target effect-site concentrations of remifentanil (R2=2 ng/mL, R4=4 ng/mL, R6=6 ng/mL). Two patients in group R4 and three patients in group R6 did not receive allocated intervention because their SBP were greater than 160 mm Hg.
Patients were randomly assigned to three groups and assignment was concealed in an envelope until the patient's entrance into the operating room. And independent observer took an envelope and prepared the target controlled infusion pump (Orchestra®, Fresinius Vial, France) for the infusion of remifentanil.
Premedication was not provided and patients were monitored with continuous electrocardiography, pulse oximetry and automated noninvasive blood pressure measurements. Patients were allowed to rest prior to baseline BP and HR measurements.
After the injection of lidocaine 30 mg and etomidate 0.2 mg/kg, remifentanil was infused in target effect-site concentrations of 2 ng/mL (Group R2, n=23), 4 ng/mL (Group R4, n=21) and 6 ng/mL (Group R6, n=20). The Minto model was used for remifentanil pharmacokinetic parameters. After confirmation of a loss of consciousness, rocuronium 0.6 mg/kg was injected. A semiclosed circle system was used with ventilation controlled by mask to maintain normocarbia as measured with end-tidal carbon dioxide (ETCO2) level. Oxygen flow rates were maintained at 5 L/min throughout this study.
After remifentanil reached the target effect-site concentration, desflurane vaporizer was immediately advanced to 2 minimum alveolar concentrations (MAC) (Table 1) and controlled ventilation continued. The vaporizer was then adjusted so that the end-tidal desflurane concentration could be maintained at 1.7 MAC for 5 minutes (over-pressure paradigm was employed). In previous reports regarding the cardiovascular stimulation effects of desflurane, the same vol% of desflurane was inhaled by all patients regardless of age.7,14 Since MAC of an inhaled anesthetic may differ depending on age, the MAC of desflurane was calculated depending on the age of each patient in this study.15,16 A gas analyzer (Smart Anesthesia Multi-gas Module™, General Electric-Marquette Medical Systems, Milwaukee, WI, USA) was used to measure the ETCO2 and end-tidal desflurane concentrations.
This study was double-blinded. To minimize investigator bias, the desflurane inhalation procedure was performed by one anesthesiologist who did not know the effect-site concentration of the infused remifentanil. SBP, diastolic blood pressure (DBP), mean arterial pressure (MAP), HR and end-tidal desflurane concentration were measured at 1 minute intervals during the 5 minutes of desflurane inhalation by another independent observer.
The experiment was stopped for appropriate treatments when any of the following side effects developed: 1) MAP less than 50 mm Hg or SBP greater than 190 mm Hg; 2) HR greater than 130 beats per minute or less than 40 beats per minute; 3) MAP greater than 110 mm Hg 5 minutes after inhalation; 4) development of arrhythmia; 5) cases where proper ventilation was not possible (i.e., laryngospasm).
Patients were intubated 5 minutes after desflurane inhalation.
Sample size calculation was done by G* Power (Ver 3.2.1, Kiel, Germany). Through the preliminary tests of five patients per each group, the mean and standard deviation (SD) of SBP and HR were calculated. Using these values, total sample size was calculated as 45 (15 in each group) for the effect size f of 0.49, a power of 80%, and a level of significance of 0.05. Assuming a 50% dropout rate, 23 patients in each group were needed.
SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses and all data were expressed as the mean±SD. Age, weight and height among groups were analyzed with one-way analysis of variance (ANOVA) and chi-square tests were performed to investigate differences of sex ratios among groups. Shapiro-Wilk tests were used to analyze the distribution of BP, HR, and MAC. A Friedman tests were performed to compare HR according to time and a Wilcoxon-signed rank test with Bonferroni correction for changes of HR as compared with baseline HR within group was performed. A Kruskal-Wallis test was used for comparison of HR among groups at specific time. Repeated measures ANOVA was used for the comparisons of BP and MAC including main effects of remifentanil groups as a between-subject variables and time points of measurements as within-subject variables, and time-group interaction. The sphericity assumption was tested using a Mauchly's test for sphericity and in cases where the sphericity assumption was significantly violated; a Greenhouse-Geisser adjustment of degree of freedom was used. One way ANOVA with a Duncan test as a post-hoc test was used for the comparisons of BP and MAC among groups. Fisher's exact tests were used to compare development frequencies of side effects. p-values less than 0.05 were considered statistically significant.
Sixty-nine patients were recruited for this study and randomly allocated into 3 groups. Two and three patients in group R4 and R6, respectively, did not receive allocated intervention due to exclusion criteria. Eighteen, twenty, and seventeen patients in group R2, R4, and R6, respectively, were included in analysis (Fig. 1).
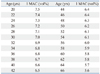
There were no significant differences in age, sex, weight and height among 3 groups (Table 2). There were no significant differences in baseline BP and HR before induction (Table 3). There were no significant differences in MAC increase after desflurane administration among the groups (Fig. 2). The mean end-tidal desflurane concentrations measured at 1, 2, 3, 4 and 5 minutes after inhalation in total patients were 1.06±0.21, 1.38±0.18, 1.50±0.13, 1.58±0.13 and 1.63±0.11 MAC.
The SBP in group R2 increased significantly compared with baseline 2 minutes after inhalation, while it decreased significantly in group R6 5 minutes after inhalation (p<0.05). Comparing the SBP among these groups, the SBP of group R2 was significantly higher than that of group R4 or R6 1 minute after inhalation. There were significant differences in SBP between groups R2, R6 and group R4 after 2 minutes of inhalation. The SBP of group R6 was significantly lower 3 minutes after inhalation compared to group R2 or R4 (p<0.05) (Fig. 3).
The DBP in group R2 increased significantly 1 and 2 minutes after inhalation compared with the baseline value and then decreased significantly 5 minutes after inhalation. The DBP decreased significantly 5 minutes after inhalation in group R6 (p<0.05). Comparing the DBP among the three groups, DBP of group R2 was significantly higher 1 and 2 minutes after inhalation than that of group R4 or R6 (p<0.05) (Fig. 4).
The MAP in group R2 increased significantly 1 and 2 minutes after inhalation compared with baseline values, but it decreased significantly 1, 2, 4 and 5 minutes after inhalation in group R6 (p<0.05). Comparing the MAP among the three groups, the MAP of group R2 was significantly higher than that of group R4 or R6, and there were significant differences in MAP 2 minutes after inhalation between groups R2, R6 and group R4 (p<0.05) (Fig. 5).
The HR in group R2 increased significantly 2 and 3 minutes after inhalation compared to baseline values (p<0.05). Among the three groups, the HR of group R2 at 1, 2 and 3 minutes after inhalation was significantly higher than that of group R4 or R6 (p<0.05) (Fig. 6).
In nine cases the experiment was stopped due to side effects. In group R2, 3 cases had a SBP greater than 190 mm Hg, 1 case with a MAP greater than 110 mm Hg at 5 minutes after inhalation, and 1 case with a HR greater than 130 beats per minute. In group R6, there were no cases with severe hypertension or tachycardia, but there were 2 cases with MAP less than 50 mm Hg and 1 case with the HR less than 40 beats per minute. There was 1 case with a SBP greater than 190 mm Hg in group R4. However, there are no significant differences in the frequency of side effects among the three groups (Table 4).
The purpose of this study was to determine the appropriate effect-site concentrations of remifentanil to attenuate cardiovascular response that could develop after inhalation of high concentration desflurane. When remifentanil was administered with an effect-site concentration of 4 ng/mL, SBP, DBP, MAP and HR did not significantly change compared with the baseline values measured before induction. The experiment was stopped in only one patient (4.8%) in group R4 and this was the lowest rate among the 3 groups. When remifentanil was administered with an effect-site concentration of 2 ng/mL, SBP, DBP, MAP and HR increased significantly compared with baseline values. The tachycardia or hypertension that was profound enough to discontinue the experiment occurred in 5 of 23 cases (21.7%). Given these findings, it can be assumed that remifentanil at 2 ng/mL did not effectively block increases in SBP, DBP, MAP and HR induced by desflurane. On the other hand, administration of 6 ng/mL decreased SBP, DBP and MAP significantly compared to baseline values. The number of cases that required cessation of the experiment due to hypotension or bradycardia was 3 out of 20 cases (15%). Based on these results, it appears that 6 ng/mL may not be the proper remifentanil concentration for the maintenance of hemodynamic stability. However, no significant differences in the number of patients with side effects were noted among these groups which may be partly due to our small sample sizes.
In this study, BP and HR showed significant differences among the 3 groups during the initial 3 minutes of desflurane inhalation at about 1 to 1.5 MAC. Our study showed that remifentanil 4 ng/mL is a proper effect-site concentration for the initial 1-3 minutes and there is no clinical difference if 2 ng/mL or 4 ng/mL of remifentanil is administered after the first 3 minutes.
Ahn, et al.17 reported that remifentanil effectively prevented not only cardiovascular adverse reactions, but also airway response during induction with desflurane. In this study, there were no severe adverse reactions in the respiratory system such as laryngospasm for all groups.
Desflurane induces airway irritation around 1 MAC and sympathetic activation above 1 MAC.7,18-20 In previous reports on the cardiovascular stimulation effect of desflurane, 10-11 vol% desflurane corresponding to approximately 1.7 MAC was used.7,14 However, the MAC of a volatile anesthetic agent may vary depending on body temperature, age, concurrent use of pharmacologic agents (such as alcohol, amphetamine, opioid, ketamine) as well as BP and pregnancy.15 It is well known that MAC decreases with age.16 Thus, inhalation of desflurane with the same concentration without an age consideration may lead to desflurane inhalation at different MAC.
This study attempted to examine the cardiovascular stimulation effect of desflurane with the administration of 1.7 MAC, which would be the MAC-beta-adrenergic response (MAC-BAR). MAC-BAR (1.7-2.0 MAC) is the concentration of inhaled anesthetic required to block the autonomic responses to nociceptive stimuli.21 In our study, no differences in age corrected MAC of end tidal desflurane concentration among the groups were noted. Since the same MAC of desflurane is achieved in each group, the differences of hemodynamic parameters among the groups may be determined by a concurrent effect-site concentration of remifentanil.
Concurrent administration of remifentanil with propofol or an inhalation anesthetic may effectively suppress hemodynamic change during surgery without increasing postoperative complications and enable rapid recovery and early extubation.12,22,23
It is appropriate to provide a combined administration of desflurane and remifentanil in the following circumstances: 1) cases where meticulous titration is necessary, especially in patients with underlying cardiovascular disorders; 2) cases where prevention of postoperative respiratory depression is required given compromised respiratory function; 3) cases where prolonged duration of anesthetics is expected due to impaired drug metabolism and excretion in patients with hepatic or renal disease; 4) cases of out-patient procedures where rapid emergence and recoveries are needed.12,24
This study did not have a control group without the remifentanil administration because the previous studies documented increases in BP and HR by inhalation of desflurane only at high concentrations.7,14 Kim, et al.14 reported that inhalation of high concentrations of desflurane (end-tidal concentration 10 vol%) led to a significant increase in SBP and DBP as well as the HR in ASA physical status class I patients. Ebert and Muzi7 reported that increasing the concentration of desflurane from 1.0 MAC (7.25 vol%) to 1.5 MAC (10.9 vol%) led to hypertension and tachycardia due to sympatho-excitation.
Hemodynamic changes following desflurane inhalation may be different depending on age. In 20 to 50-year-old patients, the most severe changes may develop 60-150 seconds after desflurane inhalation and remarkable rises may continue for 3-4 minutes.7,14 In our investigation, cardiovascular responses and adverse reactions were observed over the 5 minutes after desflurane inhalation to include the period of the most profound hemodynamic change. However, clinical intubation is performed when muscle relaxation by rocuronium is sufficiently achieved (1.7 minutes).25 Thus, the results up to 3 minutes in this study may be applied to the anesthetic induction by desflurane inhalation. When 1.7 MAC desflurane is inhaled during induction, the appropriate effect-site concentration of remifentanil to prevent hemodynamic changes is 4 ng/mL.
In this study, etomidate was used as an anesthetic induction agent. In previous reports, researchers attempted to eliminate the cardiovascular effects of thiopental sodium by initiating inhalation of desflurane 2 minutes after an infusion of thiopental sodium.7,14 Unlike thiopental sodium or propofol, etomidate has less effect on the cardiovascular and respiratory system after infusion of an anesthetic induction dosage.11 Therefore, we minimized the effects of an induction agent on BP and HR by administrating etomidate.
This study has several limitations. First, we included only relatively young and healthy patients under 65 years of age. Cardiovascular responses following the inhalation of high concentration desflurane may be different depending on age. A previous study showed that the rate of increase in MAP among patients over 65 years of age was higher than that of other age groups.14 There was a significant number of patients who had been excluded from the study due to severe increases in BP in elderly patients as compared to other age groups. This may suggest that an experiment targeting elderly patients might have different results. Furthermore, patients with cardiovascular disorders may present with more severe hemodynamic changes. Accordingly, further studies focusing on elderly patients or patients with a cardiovascular disease are needed.
Second, epinephrine and norepinephrine, which reflect responses of the sympathetic nervous system following desflurane inhalation, were not measured. It has not been elucidated whether or not the effect-site concentration of 4 ng/mL of remifentanil, required for hemodynamic stability, diminishes the responses of the sympathetic nervous system. Evidences that fentanyl and alfentanil may attenuate hemodynamic responses without blunting sympathetic nervous system activation suggest the need for further research on effects of sympathetic nervous system suppression by remifentanil.8.9
Third, our study has no control group. However, there was no need for a control group because previous reports showed significant cardiovascular stimulation with desflurane inhalation at high concentrations.7,14
Fourth, we selected 2, 4, and 6 ng/mL of remifentanil as effect-site concentrations considering that effect-site concentrations of 5 ng/mL and 2 ng/mL were effective in blunting sympathetic responses to tracheal intubation and skin incision, respectively, in 50% of patients.26 However, more accurate and appropriate effect-site concentrations of remifentanil to suppress the cardiovascular stimulation induced by high concentration of desflurane must be determined by further investigation using Dixon's up-and-down method.27
In conclusion, this prospective, randomized double-blind study demonstrates that, when compared with 2 or 6 ng/mL, 4 ng/mL of remifentanil may be the most appropriate and clinically useful effect-site concentration to prevent hemodynamic instability caused by inhalation of high concentration desflurane (1.7 MAC) because there were no significant hemodynamic changes from the baseline values.
Figures and Tables
 | Fig. 1CONSORT flow chart showing participant disposition. SBP, systolic blood pressure; MAP, mean arterial pressure; HR, heart rate; CONSORT, consolidated standards of reporting trials. |
 | Fig. 2Changes in MAC value during desflurane inhalation. There were no significant differences among the groups. MAC, minimum alveolar concentration. |
 | Fig. 3Changes in SBP during desflurane inhalation. *p<0.05 compared with baseline value within groups, †p<0.05 compared with R2 group, ‡p<0.05 compared with R4 group. SBP, systolic blood pressure. |
 | Fig. 4Changes in DBP during desflurane inhalation. *p<0.05 compared with baseline value within groups, †p<0.05 compared with R2 group. DBP, diastolic blood pressure. |
 | Fig. 5Changes in MAP during desflurane inhalation. *p<0.05 compared with baseline value within groups, †p<0.05 compared with R2 group, ‡p<0.05 compared with R4 group. MAP, mean arterial pressure. |
 | Fig. 6Changes in HR during desflurane inhalation. *p<0.05 compared with baseline value within groups, †p<0.05 compared with R2 group. HR, heart rate. |
References
2. Patel SS, Goa KL. Desflurane. A review of its pharmacodynamic and pharmacokinetic properties and its efficacy in general anaesthesia. Drugs. 1995. 50:742–767.
3. Morgan GE, Mikhail MS, Murray MJ. Strauss M, Lebowitz H, Boyle PJ, editors. Inhalation anesthetics. Clinical Anesthesiology. 2006. 4th ed. New York: McGraw Hill Inc;155–178.
4. Bennett JA, Lingaraju N, Horrow JC, McElrath T, Keykhah MM. Elderly patients recover more rapidly from desflurane than from isoflurane anesthesia. J Clin Anesth. 1992. 4:378–381.

5. Dupont J, Tavernier B, Ghosez Y, Durinck L, Thevenot A, Moktadir-Chalons N, et al. Recovery after anaesthesia for pulmonary surgery: desflurane, sevoflurane and isoflurane. Br J Anaesth. 1999. 82:355–359.

6. Sutton TS, Koblin DD, Gruenke LD, Weiskopf RB, Rampil IJ, Waskell L, et al. Fluoride metabolites after prolonged exposure of volunteers and patients to desflurane. Anesth Analg. 1991. 73:180–185.

7. Ebert TJ, Muzi M. Sympathetic hyperactivity during desflurane anesthesia in healthy volunteers. A comparison with isoflurane. Anesthesiology. 1993. 79:444–453.

8. Weiskopf RB, Eger EI 2nd, Noorani M, Daniel M. Fentanyl, esmolol, and clonidine blunt the transient cardiovascular stimulation induced by desflurane in humans. Anesthesiology. 1994. 81:1350–1355.

9. Yonker-Sell AE, Muzi M, Hope WG, Ebert TJ. Alfentanil modifies the neurocirculatory responses to desflurane. Anesth Analg. 1996. 82:162–166.

10. Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B). Anesth Analg. 1993. 77:1031–1040.
11. Morgan GE, Mikhail MS, Murray MJ. Strauss M, Lebowitz H, Boyle PJ, editors. Nonvolatile anesthetic agents. Clinical Anesthesiology. 2006. 4th ed. New York: McGraw Hill Inc;179–204.
12. Song D, White PF. Remifentanil as an adjuvant during desflurane anesthesia facilitates early recovery after ambulatory surgery. J Clin Anesth. 1999. 11:364–367.

13. Gesztesi Z, Mootz BL, White PF. The use of a remifentanil infusion for hemodynamic control during intracranial surgery. Anesth Analg. 1999. 89:1282–1287.

14. Kim EA, Kim SK, Lim HS, Ko SH, Han YJ, Song HS. The effect of age on the cardiovascular responses induced by inhaled high concentration of desflurane. Korean J Anesthesiol. 2007. 53:435–440.

15. Stanski DR, Shafer SL. Miller RD, editor. Measuring depth of anesthesia. Miller's Anesthesia. 2006. 6th ed. Philadelphia: Churchill Livingstone Inc;1227–1264.
17. Ahn ST, Lee JH, Cheong SH, Lee KM, Lee SE, Kim YH, et al. The effect of continuous remifentanil infusion on the airway reactivity during desflurane inhalation. Korean J Anesthesiol. 2007. 53:448–452.

18. Rampil IJ, Lockhart SH, Zwass MS, Peterson N, Yasuda N, Eger EI 2nd, et al. Clinical characteristics of desflurane in surgical patients: minimum alveolar concentration. Anesthesiology. 1991. 74:429–433.
19. Van Hemelrijck J, Smith I, White PF. Use of desflurane for outpatient anesthesia. A comparison with propofol and nitrous oxide. Anesthesiology. 1991. 75:197–203.

20. Moore MA, Weiskopf RB, Eger EI 2nd, Noorani M, McKay L, Damask M. Rapid 1% increases of end-tidal desflurane concentration to greater than 5% transiently increase heart rate and blood pressure in humans. Anesthesiology. 1994. 81:94–98.

21. Cheung AT, Marshall BE. Longnecker DE, Murphy FL, editors. The inhaled anesthetics. Dripps/Eckenhoff/Vandam Introduction to anesthesia. 1997. 9th ed. Philadelphia: W.B. Saunders Inc;75–87.
22. Philip BK, Scuderi PE, Chung F, Conahan TJ, Maurer W, Angel JJ, et al. The Remifentanil/Alfentanil Outpatient TIVA Group. Remifentanil compared with alfentanil for ambulatory surgery using total intravenous anesthesia. Anesth Analg. 1997. 84:515–521.

23. Song D, Whitten CW, White PF. Remifentanil infusion facilitates early recovery for obese outpatients undergoing laparoscopic cholecystectomy. Anesth Analg. 2000. 90:1111–1113.

24. Tonner PH, Scholz J. Total intravenous or balanced anaesthesia in ambulatory surgery? Curr Opin Anaesthesiol. 2000. 13:631–636.

25. Naguib M, Lien CA. Miller RD, editor. Pharmacology of muscle relaxants and their antagonists. Miller's Anesthesia. 2006. 6th ed. Philadelphia: Churchill Livingstone Inc;481–572.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download