Abstract
Purpose
It is to examine clinical manifestations, early biochemical indicators, and risk factors for non-oliguric hyperkalemia (NOHK) in extremely low birth weight infants (ELBWI).
Materials and Methods
We collected clinical and biochemical data from 75 ELBWI admitted to Ajou University Hospital between Jan. 2008 and Jun. 2011 by reviewing medical records retrospectively. NOHK was defined as serum potassium ≥7 mmol/L during the first 72 hours of life with urine output ≥1 mL/kg/h.
Results
NOHK developed in 26.7% (20/75) of ELBWI. Among NOHK developed in ELBWI, 85% (17/20) developed within postnatal (PN) 48 hours, 5% (1/20) experienced cardiac arrhythmia and 20% (4/20) of NOHK infants expired within PN 72 hours. There were statistically significant differences in gestational age, use of antenatal steroid, and serum phosphorous level at PN 24 hours, and serum sodium, calcium, and urea levels at PN 72 hours between NOHK and non-NOHK groups (p-value <0.050). However, there were no statistical differences in the rate of intraventricular hemorrhage, arrhythmia, mortality occurred, methods of fluid therapy, supplementation of amino acid and calcium, frequencies of umbilical artery catheterization and urine output between the two groups.
Conclusion
NOHK is not a rare complication in ELBWI. It occurs more frequently in ELBWI with younger gestational age and who didn't use antenatal steroid. Furthermore, electrolyte imbalance such as hypernatremia, hypocalcemia and hyperphosphatemia occurred more often in NOHK group within PN 72 hours. Therefore, more use of antenatal steroid and careful control by monitoring electrolyte imbalance should be considered in order to prevent NOHK in ELBWI.
Hyperkalemia is a frequently observed electrolyte imbalance in premature infants with a gestational age <28 weeks during the first days of life.1 Hyperkalemia which develops in the absence of oliguria and potassium intake is known as non-oliguric hyperkalemia (NOHK).2
In extremely premature infants, early-onset of NOHK is common and can cause serious complications.3,4 Although it is believed that premature infants may well tolerate severe hyperkalemia, it may cause fatal cardiac arrhythmia, periventricular leukomalacia, brain hemorrhage, and even sudden death.5-8 The reported incidence of NOHK varies widely from 0%9 to 60%.9-14 The reason for this difference may be related to different population groups and different level of thresholds used for defining hyperkalemia.
Although extreme prematurity might be considered as a primary risk factor for NOHK,12,13,15,16 no other risk factors have yet been clearly described.12,17,18 We, therefore, performed this study to examine clinical manifestations, early biochemical indicators, and risk factors associated with NOHK in extremely low birth weight infants (ELBWI).
We retrospectively reviewed the medical records of all infants whose birth weights were less than 1000 g and were admitted to the Neonatal Intensive Care Unit of Ajou University Hospital between January 2009 and June 2011. Infants with major congenital anomalies were excluded.
All infants started to receive electrolyte-free fluids after insertion of umbilical catheters. And sodium was added after the first urination, while potassium was withheld for the first 72 hours of life. Amino acid supplementation (primene®) was followed, according to recommended dietary intake for preterm infants.19-21 Seventy-five ELBWI were enrolled in this study. This study was approved by the institutional Review Board at the Ajou University Hospital.
We collected clinical data including gestational age, birth weight, Apgar scores at 1 and 5 minutes of life, gender, mortality, use of antenatal steroid, and existence of severe bruising at birth. Respiratory distress syndrome (RDS), patent ductus arteriosus (PDA), cardiac arrhythmia, intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC) and retinopathy of prematurity (ROP) were also recorded.
Serum electrolytes were measured within 2 hours of birth (pH, sodium, potassium), at postnatal (PN) 24 hours (pH, sodium, potassium, calcium, phosphate) and PN 72 hours (pH, sodium, potassium, calcium, phosphate, urea, creatinine, albumin). Unit policy was to sample blood from an indwelling catheter (mainly, umbilical arterial catheter) or by arterial puncture to prevent hemolysis. In all cases, the following data were recorded: supplementation of amino acid and calcium, daily weight, infusion of dopamine, insertion of umbilical arterial catheter, amount of daily fluid, hourly urine output, and daily body weight change.
Patients were identified with NOHK if they had a serum potassium ≥7 mmol/L during the first 72 hours of life with urine output ≥1 mL/kg/h.12 Bronchopulmonary dysplasia was defined as supplementation of oxygen needed at 36 weeks' postmenstrual age,22 NEC defined as Bell's stage Ib or greater,23 and IVH defined as Papile's classification II or greater.24
SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The χ2 or Fisher's exact test was used to compare categorical variables, and the Student's t-test and the Mann-Whitney's rank sum U test were used to compare continuous variables in univariate analysis. Logistic regression modeled factors were identified as significant in the multivariate analysis. Categorical data are presented as percentages (%), and continuous data as mean±SD or median (25-75%), and we rejected the null hypothesis when p-value was less than 0.050.
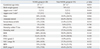
Seventy-five ELBWI (38 males and 37 females) met the study criteria, and 20 (26.7%) were diagnosed as NOHK. NOHK developed almost within PN 48 hours in 17 (85%) of 20 NOHK diagnosed ELBWI.
There were significant differences in gestational age and use of antenatal steroid between NOHK group and non-NOHK group (p-value 0.019 and 0.001, respectively). However, other perinatal characteristics, morbidities, cardiac arrhythmia, mortality, supplementation of amino acid and calcium, dopamine infusion, insertion of umbilical artery catheterization (UAC), fluid therapy, hourly urine output and daily changes of body weight failed to show such meaningful differences between the groups.
In the medical records, cardiac arrhythmias were documented in 1 (5%) of the NOHK infants and 2 (3.6%) of the non-NOHK infants. Type of arrhythmia developed in NOHK group was Tall T wave, which was detected early by electrocardiography monitoring and corrected soon after anthertihyperkalemic treatment (sodium bicarbonate, calcium gluconate, or insulin/dextrose). In non-NOHK group, sinus arrhythmia and premature ventricular contraction were shown, however, spontaneously disappeared without specific managements.
The biochemical indicators were compared between the groups and date are shown in Table 4.
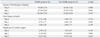
In blood samples taken at PN 24 hour, serum potassium (p-value <0.001) and phosphorous (p-value=0.024) levels were found to have increased significantly in NOHK group. Serum sodium and calcium levels were similar in both groups.
At PN 72 hours, NOHK infants showed higher serum sodium (p-value=0.011) and serum urea (p-value=0.010) levels, and showed lower serum calcium (p-value=0.033) level compared with non-NOHK infants. Serum pH, phosphorous, creatinine, and albumin levels showed no significant differences between the groups.
Multivariate analysis indicated no statistically significant risk factor among the biochemical indicators listed in Table 4.
Hyperkalemia is often observed transiently in ELBWI, with a maximum level reaching in PN 12 and 24 hours.25 This reversible condition does not appear to be associated with renal failure, increased potassium intake, or excessive bruising.9 There are several mechanisms suggested for early hyperkalemia, including poor urinary potassium excretion, release of potassium from lysed red cells in bruised infant, or a shift of potassium from the intracellular space to the extracellular space due to an immature function of the Na+/K+-adenosine triphosphatase (ATPase) activity.15,16
The incidence of NOHK in this study was 26.7% of ELBWI which is consistent with previously reported studies.9-14 Although all infants in this study were below 28 weeks of gestational age, infants in NOHK group were significantly younger than non-NOHK group. Theoretically, younger infants may have less matured functional Na+/K+-ATPase activity in extreme prematurity,15,16 and this could be a plausible explanation of NOHK of our study group.
In this study, we demonstrated that antenatal steroid therapy was less common in NOHK group than non-NOHK group. Consistent with our data, Omar and others reported that administration of prenatal steroid not only reduces the risk of IVH, RDS, and NOHK, but also is effective in protection of hyperkalemia by increasing the activity of Na+/K+-ATPase in ELBWI.26-29
Hung found a 12% risk of cardiac arrhythmia in ELBW infants with NOHK,30 and up to 60% was reported in other studies.5,26 In our present study, 1 (5%) of 20 ELBW infants with NOHK developed cardiac arrhythmia, and 4 (20%) of 20 NOHK infants expired with PN 72 hours. However, there were no significant differences in the risk of cardiac arrhythmia and mortality between the two groups. This might be due to our early detection and treatment strategy for hyperkalemia or remarkable tolerance of the myocardium to hyperkalemia in some extremely preterm infants.31 De Luca and Paolillo31 reported the absence of arrhythmias in an ELBW infant even though serum potassium was 11 mEq/L.
We did not find any association with birth weight,4 insertion of UAC and IVH (≥grade II),5,17 Apgar score, gender, brusing at birth, RDS, PDA, NEC (≥stage Ib), ROP (≥stage II), fluid therapy, urine output, and use of amino acid and dopamine,17 although several studies4,5,17 described the association of such factors with hyperkalemia. The lack of association is presumably due to the fact that all infants in this study were at high risk of NOHK (birth weight <1000 g).
Infants with NOHK had significantly increased serum phosphorus at PN 24 hours, and decreased serum calcium and increased serum sodium and urea at PN 72 hours. Fukuda found that hypocalcemia was more common in preterm infant who had serum potassium >7 mmol/L,32 and Iijima, et al.33 reported a negative correlation between the plasma potassium and ionized calcium levels at PN 12 and 24 hours, concluding that NOHK may be attenuated by maintaining the ionized calcium level within normal limits by prophylactic calcium administration early in life. However, we could find no statistically significant difference in calcium administration between NOHK and non-NOHK groups.
The limitations of this study include unequal and small group size. Further research with a large number of preterm infants, who have NOHK, is needed to better understand the significant demographic characteristics and electrolyte disturbances.
In conclusion, NOHK is not a rare complication in ELBW infants, but more common in ELBW infants with younger gestational age and without the use of antenatal steroid. There were also electrolyte imbalances such as hyperphophatemia, hypernatremia, and hypocalcemia, and increased serum urea within PN 72 hours. Therefore, more use of antenatal steroid and careful control by way of monitoring electrolyte imbalance should be considered in order to prevent NOHK in ELBW infants.
Figures and Tables
ACKNOWLEDGEMENTS
This work was supported by the new faculty research fund of Ajou University School of Medicine.
References
1. Apitz C, Wirbelauer J. [Circulatory failure due to severe cardiac arrhythmia as a result of hyperkalemia in a very low birth weight infant]. Klin Padiatr. 2006. 218:16–19.
2. Gruskay J, Costarino AT, Polin RA, Baumgart S. Nonoliguric hyperkalemia in the premature infant weighing less than 1000 grams. J Pediatr. 1988. 113:381–386.

3. Vemgal P, Ohlsson A. Interventions for non-oliguric hyperkalaemia in preterm neonates. Cochrane Database Syst Rev. 2007. CD005257.

4. Yuan HC, Jeng MJ, Soong WJ, Chen SJ, Hwang BT. Hyperkalemia during the early postnatal days in premature infants. Acta Paediatr Taiwan. 2003. 44:208–214.
5. Shortland D, Trounce JQ, Levene MI. Hyperkalaemia, cardiac arrhythmias, and cerebral lesions in high risk neonates. Arch Dis Child. 1987. 62:1139–1143.

6. Fiona M, O'Hare EJM. Clinical pattern recognition in the diagnosis of severe hyperkalemia in preterm infant. 2007. In : Pediatric Academic Society Meeting; Toronto.
7. Singh D, Dutta S, Narang A. Hyperkalemia and ventricular tachycardia in ELBW infant. Indian Pediatr. 2003. 40:64–66.
8. Wren C. Hyperkalaemia, cardiac arrhythmias, and cerebral lesions in high risk neonates. Arch Dis Child. 1988. 63:681–682.

9. Mildenberger E, Versmold H. [Results of a National Survey in Germany on incidence and therapy of the nonoliguric hyperkalemia of the premature infant]. Z Geburtshilfe Neonatol. 2002. 206:9–14.
10. Usher R. The respiratory distress syndrome of prematurity. I. Changes in potassium in the serum and the electrocardiogram and effects of therapy. Pediatrics. 1959. 24:562–576.
11. Leslie GI, Carman G, Arnold JD. Early neonatal hyperkalaemia in the extremely premature newborn infant. J Paediatr Child Health. 1990. 26:58–61.

12. Brion LP, Schwartz GJ, Campbell D, Fleischman AR. Early hyperkalaemia in very low birthweight infants in the absence of oliguria. Arch Dis Child. 1989. 64:270–272.

13. Mildenberger E, Versmold HT. Pathogenesis and therapy of non-oliguric hyperkalaemia of the premature infant. Eur J Pediatr. 2002. 161:415–422.

14. Lorenz JM, Kleinman LI, Markarian K. Potassium metabolism in extremely low birth weight infants in the first week of life. J Pediatr. 1997. 131(1 Pt 1):81–86.

15. Stefano JL, Norman ME, Morales MC, Goplerud JM, Mishra OP, Delivoria-Papadopoulos M. Decreased erythrocyte Na+,K(+)-ATPase activity associated with cellular potassium loss in extremely low birth weight infants with nonoliguric hyperkalemia. J Pediatr. 1993. 122:276–284.

16. Sato K, Kondo T, Iwao H, Honda S, Ueda K. Internal potassium shift in premature infants: cause of nonoliguric hyperkalemia. J Pediatr. 1995. 126:109–113.

17. Shim JW, Ko SY, Kim SS, Kim MJ, Chang YS, Park WS. Non-oliguric hyperkalemia in extremely low birth weight infants. J Korean Soc Neonatol. 2002. 9:21–28.
18. Yaseen H. Nonoliguric hyperkalemia in neonates: a case-controlled study. Am J Perinatol. 2009. 26:185–189.

19. American Academy of Pediatrics Committee on Nutrition: nutritional needs of low-birth-weight infants. Pediatrics. 1985. 75:976–986.
20. Ziegler EE, Thureen PJ, Carlson SJ. Aggressive nutrition of the very low birthweight infant. Clin Perinatol. 2002. 29:225–244.

21. Rigo J, Curtis MD. Guandalini S, editor. Parenteral nutrition in premature infants. Textbook of Pediatric Gastroenterology and nutrition. 2004. 1st ed. Talyor & Francis Group;619–638.
22. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988. 82:527–532.

23. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986. 33:179–201.

24. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978. 92:529–534.

25. Kilbride HW, Cater G, Warady BA. Early onset hyperkalemia in extremely low birth weight infants. J Perinatol. 1988. 8:211–214.
26. Omar SA, DeCristofaro JD, Agarwal BI, LaGamma EF. Effect of prenatal steroids on potassium balance in extremely low birth weight neonates. Pediatrics. 2000. 106:561–567.

27. Uga N, Nemoto Y, Ishii T, Kawase Y, Arai H, Tada H. Antenatal steroid treatment prevents severe hyperkalemia in very low-birth-weight infants. Pediatr Int. 2003. 45:656–660.

28. Vasarhelyi B, Tulassay T, Ver A, Dobos M, Kocsis I, Seri I. Developmental changes in erythrocyte Na(+),K(+)-ATPase subunit abundance and enzyme activity in neonates. Arch Dis Child Fetal Neonatal Ed. 2000. 83:F135–F138.

29. Scholle S, Bräunlich H. Effects of prenatally administered thyroid hormones or glucocorticoids on maturation of kidney function in newborn rats. Dev Pharmacol Ther. 1989. 12:162–168.

30. Hung KC, Su BH, Lin TW, Peng CT, Tsai CH. Glucose-insulin infusion for the early treatment of non-oliguric hyperkalemia in extremely-low-birth-weight infants. Acta Paediatr Taiwan. 2001. 42:282–286.
31. De Luca D, Paolillo P. Absence of arrhythmias in the extremely preterm heart with severe hyperkalaemia. Resuscitation. 2009. 80:961.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download