Abstract
Purpose
The purpose of this study was to evaluate the usefulness of convex probe endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) for detecting malignancy in parenchymal pulmonary lesions located adjacent to the central airways.
Materials and Methods
We retrospectively reviewed the diagnostic performance of EBUS-TBNA in consecutive patients with high clinical suspicion of a centrally located primary lung cancer who had undergone EBUS-TBNA at the Samsung Medical Center between May 2009 and June 2011.
Results
Thirty-seven patients underwent EBUS-TBNA for intrapulmonary lesions adjacent to the central airways. Seven lesions were located adjacent to the trachea and 30 lesions were located adjacent to the bronchi. Cytologic and histologic samples obtained via EBUS-TBNA were diagnostic in 32 of 37 (86.4%) of patients. The final diagnosis was lung cancer in 30 patients (7 small cell lung cancer, 23 non-small cell lung cancer), lymphoma in one and malignant fibrous histiocytoma in one patient. The diagnostic sensitivity of EBUS-TBNA in detecting malignancy and detecting both malignancy and benignity was 91.4% and 86.5%, respectively. Two patients experienced minor complications.
Lung cancer is the leading cause of cancer related deaths worldwide, with an estimated one to two million deaths each year.1 Lung cancer causes more deaths than the next four most common cancers combined, including cancer of the breast, colon, pancreas and prostate.1 If lung cancer is suspected, a histological diagnosis, in conjunction with accurate staging, is needed in order to guide therapy and prognosis.2,3
The conventional methods for establishing diagnosis of intraparenchymal lesions are transthoracic needle aspiration (TTNA) or transbronchial needle aspiration/biopsy by bronchoscopy with or without fluoroscopy. Other newer techniques such as computed tomography (CT) guided bronchoscopy,4 virtual bronchoscopy,5,6 electromagnetic navigation bronchoscopy7,8 and endobronchial ultrasound guide-sheath9,10 have been developed to improve diagnostic yield, but have not become mainstream modalities and are only available at limited centers.
Convex probe endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has been shown to be useful for staging of the mediastinum with high sensitivity, and when combined with endoscopic ultrasound, accessing lymph node stations inaccessible even by mediastinoscopy, enabling near complete mediastinal evaluation.11-14
Although the utility of EBUS-TBNA to diagnose parenchymal lesions in cases of non-diagnostic conventional bronchoscopy is being recognized and emerging, there are limited reports about its application for this purpose.15-17 The purpose of this study was to evaluate the usefulness of EBUS-TBNA for detecting malignancy in the parenchymal lesions located adjacent to the central airways.
We retrospectively reviewed the diagnostic performance of EBUS-TBNA in consecutive patients with high clinical suspicion of a centrally located primary lung cancer who had undergone EBUS-TBNA at the Samsung Medical Center between May 2009 and June 2011. The centrally located lung lesions were defined as an intrapulmonary nodule or mass located adjacent to the tracheobronchial tree as visualized on chest CT scan. This study was approved by the Institutional Review Board of the Samsung Medical Center, which waived the requirements for informed consent of the individual patients, given the retrospective nature of the study.
EBUS-TBNA was performed by three trained operators using a curvilinear scanning ultrasound bronchoscope (BF UC260F-OL8, Olympus Ltd., Tokyo, Japan) connected to an ultrasound unit (EU-C60 Olympus Ltd., Tokyo, Japan). The procedures were performed under local anesthesia (lignocaine) and moderate sedation (midazolam). For paratracheal lesions, the scope was positioned endotracheally. For peribronchial lesions the scope was positioned in the respective bronchi in order to visualize the lung lesion. TBNA was performed using a 22-gauge needle (NA-2015X-4022, Olympus Ltd., Tokyo, Japan). Two aspirates were performed with 15 passes (moving needle back & forth in the lesion) per aspirate. The core tissue was expelled onto piece of paper for histological examination and the needle was flushed with saline onto glass slides for cytological examination. The aspirate was smeared onto glass slides, air dried, fixed immediately with 95% alcohol, and stained with Hematoxylin and Eosin (HE). Histological cores were fixed with 10% neutral buffered formalin and stained with HE. Rapid on-site cytological examination was not available. A post-procedure chest X-ray was routinely performed to exclude any procedure-related complications.
Cyto-pathological specimens from EBUS-TBNA were categorized as malignant (presence of malignant cells), benign (normal lung tissue without malignant cells), or inadequate (no or scanty cellular component, blood only, mucus, or benign bronchial cells only). Malignant and benign samples diagnosed by EBUS-TBNA were defined as adequate samples. Tumor-positive findings from the EBUS-TBNA samples were not surgically validated. The diagnostic standard for benign disease was pathological or microbiological confirmation of a specific benign disease, surgical confirmation of lesions showing no malignant cells, or spontaneous regression of lung parenchymal lesion after EBUS-TBNA during follow-up of more than 6 months. Malignant disease was defined as confirmation of malignant cells or tissues from EBUS-TBNA, CT-guided transthoracic needle aspiration or surgical lung biopsy. Cases which did not satisfy the criteria of benign or malignant disease were classified as indeterminate cases.
If the pathology from the EBUS-TBNA samples resulted in a diagnosis of malignancy, corroborative evidence of malignancy was looked for in the form of surgical lung biopsy or biopsy results of tissue obtained from some other site suspected of malignant involvement. The results of the concurrent EBUS-TBNA of mediastinal lymph nodes were excluded from analysis.
All data are presented as a number (%) or median (range) unless otherwise stated. Diagnostic performance analyses were performed in relation to diagnosis of malignancy and diagnosis of all disease (benignity and malignancy). Patients who had an indeterminate final diagnosis were considered a false negative result of EBUS-TBNA. The sensitivity, specificity, and diagnostic accuracy for detecting malignancy were calculated using the standard definitions.
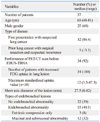
Thirty-seven patients underwent EBUS-TBNA for intrapulmonary lesions adjacent to the central airways. There were 25 (68%) males and the median age was 63 years (40-81). The size of pulmonary lesions on CT varied from 8 to 82 mm in short axis with a median of 27.5 mm (22 lesions were less than 3 cm and 15 were larger than 3 cm). Positron emission tomography (PET) scan was done in 34 (92%) patients and increased FDG uptake was seen in the parenchymal mass lesion in all patients. However, median standard uptake value (SUVmax) was only available in 29 (78%) patients as 8 patients had PET scan done outside our institution. Median SUVmax was 12 (3.3-47.3) (Table 1). Fifteen (40.5%) patients had endobronchial abnormality. Twelve (32%) had mucosal or submucosal abnormality such as exophytic tumor or hyperaemia, and 3 (8%) had extrinsic compression. Twenty-two (59%) had no endobronchial abnormality (Table 1).
Locations of primary tumors sampled by EBUS-TBNA and puncture sites are shown in Table 2. Right upper lobe lesions were targeted predominantly through the trachea, right main stem bronchus, or bronchus intermedius. Left upper lobe masses were sampled through the left upper lobar bronchus at its orifice, the distal left main bronchus and the left side of the trachea. Both lower lobar masses were targeted through the distal main bronchus, bronchus intermedius or the basal trunk. Two patients experienced complications. One developed pneumothorax requiring chest tube insertion and another patient developed moderate bleeding which resolved spontaneously. Both patients recovered from their complications uneventfully. A representative case of EBUS-TBNA for lung parenchymal lesion is described in Fig. 1.
Twenty-two out of 37 (59.4%) patients had undergone mucosal and transbronchial biopsies by conventional bronchoscopy. Out of these, 19 patients had it done at a median (range) interval of 4.5 (1-7) days prior to EBUS-TBNA and 3 patients had it done in the same sitting as EBUS-TBNA. The results of conventional bronchoscopic biopsy were non-diagnostic in 17 patients (15 patients in the prior to EBUS-TBNA group and in 2 patients in the same sitting group) with an overall non-diagnostic rate of 17/22 (77.3%). Out of 5 cases that had positive bronchoscopic biopsy, one had a slit like narrowing of the distal left main bronchus with a submucosal lesion, one had a submucosal lesion in the left upper lobe, two had mucosal abnormality in the right upper lobe and one had a left lower lobe mucosal lesion. EBUS-TBNA was performed on these patients, despite positive yield, to ascertain the cell type when the cell type was not clearly discernible (doubtful) based on conventional bronchoscopy, or to obtain additional tissue for mutation analysis (for patients who had bronchoscopy done outside our institute). Seventeen cases were judged to be non-accessible by bronchoscopic biopsies based on chest CT scans and bronchoscopic findings. Sputum was evaluated in 21 patients and was positive in one (2.7%). TTNA was done in 7 patients and was positive in one (2.7%) of these patients (Table 3).
Out of the 32 cases of malignancy detected by EBUS-TBNA, corroborative evidence of malignancy was found in the cerebrospinal fluid in 1 case, brain biopsy in 1 case, lobectomy in 1 case, tonsillectomy in 1 case, colon polypectomy in 1 case, tonsil and duodenal biopsy along with bone marrow biopsy in 1 case, pleural fluid in 1 case, thyroid fine-needle aspiration in 1 case and supraclavicular lymph node aspiration in 4 cases (Table 4).
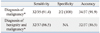
Pathologic diagnosis was achieved in 32 patients (86.4%) by EBUS-TBNA (Table 5, Fig. 2). The diagnoses established were lung cancer in 30 patients [7 small cell lung cancer (SCLC), 23 non-small cell lung cancer (NSCLC)], lymphoma in one and malignant fibrous histiocytoma in one (Table 5, Fig. 2). EBUS-TBNA was non-diagnostic in 5 patients. Out of these, one patient was diagnosed with Epstein-Barr virus (EBV) related lymphoproliferative disorder by lobectomy, one was diagnosed with tuberculosis based on positive culture of bronchoalveolar lavage fluid, one was diagnosed with benign disease due to interval radiological resolution, one was diagnosed with non-small cell lung carcinoma on bronchoscopic biopsy and one did not undergo any confirmatory test due to refusal. Accordingly, diagnoses of malignant disease, benign disease and indeterminate cases were confirmed in 34, 2, and 1 patient, respectively. Among 23 patients with a final diagnosis of NSCLC, 16 had advanced disease (14 with stage IV disease and 2 with stage IIIB disease), five had stage IIIA disease, one had stage IIB disease and one had stage IB disease. Among patients with a final diagnosis of SCLC, five had extensive disease and two had limited disease. Overall, three (8%) patients underwent surgery with curative intent. The diagnosis in these patients was EBV related lymphoproliferative disease (n=1), stage IB NSCLC (n=1) and malignant fibrous histiocytoma (n=1).
The sensitivity (95% confidence interval), specificity (95% confidence interval) and diagnostic accuracy of EBUS-TBNA for the diagnosis of malignancy was 91.4% (89.2-100), 100% (15.0-85.0) and 91.9%, respectively. The diagnostic sensitivity (95% confidence interval) of EBUS-TBNA in detecting both malignancy and benignity (all cases) was 86.5% (71.2-95.4) (Table 6).
The current study demonstrates that in addition to the well established usefulness of lymph node aspiration for mediastinal staging, EBUS-TBNA is a useful technique for establishing a diagnosis of parenchymal lesions located adjacent to the tracheobronchial tree. EBUS-TBNA provided the diagnosis of malignancy in 86.4% of cases. The sensitivity to diagnose malignancy was 91.4% and the specificity was 100%.
The diagnostic yield of EBUS-TBNA in this study was higher than that of conventional bronchoscopy reported for centrally located parenchymal lesions in the literature.18 Where the yield of bronchial lung biopsy is up to 95% in endobronchially visible lesions, it drops to 60-75% for parenchymal lesions ≥2 cm in diameter even with fluoroscopy.19 With TBNA, the yield has been reported to be 69.3% in parenchymal lesions with a mean diameter of 3.5 cm (range 0.8-8 cm) with fluoroscopy.20 However, in cases of submucosal or peribronchial lesions, biopsy forceps are unable to penetrate the wall of the tracheobronchial tree in order to reach the peribronchial lesion, and blindly performing bronchoscopic needle aspiration is risky, especially when there are no mucosal changes to guide the site of puncture.
Forceps/brush biopsy of parenchymal lesions with the help of radial probe EBUS with a guide sheath and fluoroscopy has been shown to have a diagnostic yield of 72.8%, 77% and 90% in lesions less than 2 cm, 2-3 cm and larger than 3 cm in size, respectively.10 Without fluoroscopy, the yield is even lower, especially in lesions that are less than 3 cm in size.9 Furthermore, this technique is more suitable for peripheral lesions than for central lesions, and it is not a real time procedure.
The diagnostic yield with newer techniques such as electromagnetic navigation bronchoscopy has been shown to be 59% when used alone, and 88% when used in conjunction with EBUS, in a randomized controlled trial.21 However, EBUS guided biopsy is more suitable for peripherally located lesions than central lesions, and is not real time in nature as the CT images used to guide the navigation are obtained much earlier than the actual time of performing the procedure.
TTNA offers the best diagnostic yield among needle based techniques, to the extent of 90-95%, but mainly for peripheral lesions. In cases of central lesions, needles must traverse a significant amount of lung tissue along its transthoracic route due to the distance between the chest wall and the lesion, and thus, pose a substantial risk of pneumothorax and bleeding.22,23
All of these issues can be overcome by using EBUS-TBNA, as lesions can be localized and visualized well. Surrounding structures and vessels can be identified by Doppler mode to ensure safety, and the real time nature of the procedure allows for continuous visualization of the needle throughout the lesion sampling. Additionally, specific areas within the lesion (non-necrotic) can be targeted for puncture to increase the yield if necessary.
Previously, Tournoy, et al.15 reported a sensitivity of 82% and negative predictive value of 23% for EBUS-TBNA in diagnosing centrally located parenchymal lesions, similar in location to our series. They defined central lesions as those with a medial border lying within the inner third of the hemi thorax. In another study, Nakajima, et al.16 demonstrated a sensitivity, specificity and accuracy of 94.1%, 100% and 94.3% respectively in similar group of patients. Our findings were similar to these results and help strengthen the evidence in favor of this application of EBUS-TBNA. In our study, we adopted a very conservative approach to calculate the diagnostic sensitivity of EBUS-TBNA. We considered one patient who had a negative result of EBUS-TBNA and did not undergo any confirmation by any method as a false negative result of EBUS-TBNA. The diagnostic sensitivity of EBUS-TBNA in detecting malignancy and detecting both malignancy and benignity (all cases) was 91.4% and 86.5%, respectively, in this study.
The present study clearly has limitations. First, due to its retrospective nature, "gold standard" surgical lung biopsy confirmation was not done in all patients. This was because of the unresectability of these lesions either due to advanced lung cancer, small cell lung cancer or prior lobectomy or pneumonectomy. It was judged unethical to subject these patients to a more invasive procedure when the diagnosis had been successfully established by EBUS-TBNA, and additional corroborative evidence was available in 31% of patients. Secondly, the low negative predictive value demonstrated in our study might be the result of the relatively high prevalence of lung cancer. Thirdly, the diagnostic yield of this technique for benign lesion remains to be established. A prospective and a larger study will help to overcome these issues. Another technical limitation arose due to the larger external diameter of the convex probe EBUS, which prevented its use for parenchymal lesions located adjacent to the more distal bronchial tree. Here is where radial probe EBUS will be more useful.
In conclusion, EBUS-TBNA seems to be an effective and safe method for tissue diagnosis of parenchymal lesions that lie centrally close to the airways and esophagus. EBUS-TBNA should be considered the procedure of choice for patients with such centrally located paratracheobronchial lesions without endobronchial involvement.
Figures and Tables
 | Fig. 1A representative case of EBUS-TBNA for lung parenchymal lesions. (A) CT scan of a 43-year-old male with lung cancer showing a mass in the right upper lobe adjacent to the right lateral wall of the trachea. (B) Endobronchial ultrasound image of the same lesion with the ultrasound probe placed next to the right lateral wall of the trachea. EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration. |
 | Fig. 2Diagnostic algorithm of study patients. EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; BAL, bronchoalveolar lavage; EBV, Ebstein-Barr virus. |
References
1. Alberg AJ, Ford JG, Samet JM. American College of Chest Physicians. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007. 132:3 Suppl. 29S–55S.
2. Schwartz AM, Henson DE. American College of Chest Physicians. Diagnostic surgical pathology in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007. 132:3 Suppl. 78S–93S.
3. Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC. American College of Chest Physicians. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007. 132:3 Suppl. 94S–107S.
4. Shinagawa N, Yamazaki K, Onodera Y, Miyasaka K, Kikuchi E, Dosaka-Akita H, et al. CT-guided transbronchial biopsy using an ultrathin bronchoscope with virtual bronchoscopic navigation. Chest. 2004. 125:1138–1143.

5. Asano F, Matsuno Y, Shinagawa N, Yamazaki K, Suzuki T, Ishida T, et al. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest. 2006. 130:559–566.

6. Tachihara M, Ishida T, Kanazawa K, Sugawara A, Watanabe K, Uekita K, et al. A virtual bronchoscopic navigation system under X-ray fluoroscopy for transbronchial diagnosis of small peripheral pulmonary lesions. Lung Cancer. 2007. 57:322–327.

7. Eberhardt R, Anantham D, Herth F, Feller-Kopman D, Ernst A. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest. 2007. 131:1800–1805.

8. Gildea TR, Mazzone PJ, Karnak D, Meziane M, Mehta AC. Electromagnetic navigation diagnostic bronchoscopy: a prospective study. Am J Respir Crit Care Med. 2006. 174:982–989.
9. Yoshikawa M, Sukoh N, Yamazaki K, Kanazawa K, Fukumoto S, Harada M, et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest. 2007. 131:1788–1793.

10. Kurimoto N, Miyazawa T, Okimasa S, Maeda A, Oiwa H, Miyazu Y, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004. 126:959–965.

11. Herth FJ, Eberhardt R, Vilmann P, Krasnik M, Ernst A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006. 61:795–798.

12. Herth FJ, Ernst A, Eberhardt R, Vilmann P, Dienemann H, Krasnik M. Endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes in the radiologically normal mediastinum. Eur Respir J. 2006. 28:910–914.

13. Yasufuku K, Nakajima T, Motoori K, Sekine Y, Shibuya K, Hiroshima K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest. 2006. 130:710–718.

14. Yasufuku K, Chiyo M, Koh E, Moriya Y, Iyoda A, Sekine Y, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005. 50:347–354.

15. Tournoy KG, Rintoul RC, van Meerbeeck JP, Carroll NR, Praet M, Buttery RC, et al. EBUS-TBNA for the diagnosis of central parenchymal lung lesions not visible at routine bronchoscopy. Lung Cancer. 2009. 63:45–49.

16. Nakajima T, Yasufuku K, Fujiwara T, Chiyo M, Sekine Y, Shibuya K, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of intrapulmonary lesions. J Thorac Oncol. 2008. 3:985–988.

17. Lee JE, Kim HY, Lim KY, Lee SH, Lee GK, Lee HS, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lung cancer. Lung Cancer. 2010. 70:51–56.

18. Rivera MP, Mehta AC. American College of Chest Physicians. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007. 132:3 Suppl. 131S–148S.
19. Gould MK, Fletcher J, Iannettoni MD, Lynch WR, Midthun DE, Naidich DP, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007. 132:3 Suppl. 108S–130S.
20. Gasparini S, Ferretti M, Secchi EB, Baldelli S, Zuccatosta L, Gusella P. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses. Experience with 1,027 consecutive cases. Chest. 1995. 108:131–137.

21. Eberhardt R, Anantham D, Ernst A, Feller-Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med. 2007. 176:36–41.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download