Abstract
Purpose
Previous studies have reported that over a third of cancer patients experience significant psychological distress with diagnosis and treatment of cancer. Mental adjustment to cancer as well as other biologic and demographic factors may be associated with their distress. We investigated the relationship between mental adjustment and distress in patients with thyroid cancer prior to thyroidectomy.
Materials and Methods
One hundred and fifty-two thyroid cancer patients were included in the final analysis. After global distress levels were screened with a distress thermometer, patients were evaluated concerning mental adjustment to cancer, as well as demographic and cancer-related characteristics. A thyroid function test was also performed. Regression analysis was performed to discern significant factors associated with distress in thyroid cancer patients.
Results
Our regression model was significant and explained 38.5% of the total variance in distress of this patient group. Anxious-preoccupation and helpless-hopeless factors on the mental adjustment to cancer scale were significantly associated with distress in thyroid cancer patients.
Conclusion
Negative emotional response to cancer diagnosis may be associated with distress in thyroid cancer patients awaiting thyroidectomy. Screening of mental coping strategies at the beginning of cancer treatment may predict psychological distress in cancer patients. Further studies on the efficacy of psychiatric intervention during cancer treatment may be needed for patients showing maladaptive psychological responses to cancer.
For the past two decades, the incidence of thyroid cancer has rapidly increased around the world.1-3 In Korea, the incidence has increased by about 8 times from 1999 to 2008 with an average annual increasing rate of about 25% (25.3% in men, 25.7% in women), exhibiting the highest increasing rate among cancers.4 Thankfully, the number of disease-free survivors of thyroid cancer are increasing, as thyroid cancer is usually developed at a relatively younger age than other cancers,4,5 and well differentiated thyroid cancer (DTC) is the most common type, which has a very good prognosis.1
Besides classical vital signs and pain, distress has been suggested as the sixth vital sign in cancer patients.6 Psychological distress among thyroid cancer patients is an important issue in regards to their quality of life (QoL). Although controversies concerning the management of DTC remain unresolved, most patients with thyroid cancer undergo a surgical operation followed by selective radioactive iodine remnant ablation and thyroid stimulating hormone suppressive therapy with levo-thyroxine. Previous studies reported a decline in QoL after treatment of thyroid cancer,7-9 and emotional distress was the major determinant of the decreased QoL for the patients with thyroid cancer.10
Individual psychological characteristics among patients can modulate distress and emotional symptoms. Mental adjustment to cancer and coping strategies for cancer-related problems have been considered as important determinants of distress and QoL in cancer patients.11,12 Thus, assessing psychological responses to cancer is essential to managing distress and to improving QoL in patients. Mental adjustment to cancer (MAC) can be defined as an individual patient's cognitive and behavioral response to diagnosis with cancer. Mental adjustment comprises two components including cognitive appraisal, the patient's personal perceptions of the implications of cancer, and following reactions, the patient's thought and behavior towards alleviating the threat raised with cancer diagnosis.13 Studies on mental health for thyroid cancer patients are not sufficient, as most studies on psychological distress and mental adjustment have been conducted for breast cancer patients.14,15
Usually, patients may experience distress and related emotional symptoms when they are informed of having cancer.16 Distress in thyroid cancer patients may change through the course of cancer treatment. Distress levels and mental adjustment to cancer need to be evaluated at the time of cancer diagnosis, as patients are especially vulnerable to distress in this period, and early intervention to decrease distress among cancer patients may improve their QoL throughout the treatment period. However, most studies on distress and emotional symptoms of thyroid cancer patients have been performed after completion of active cancer treatment or among disease-free survivors.10,17,18
In this study, we investigated global distress, mental adjustment to cancer, as well as demographic and cancer-related clinical characteristics in patients with thyroid cancer, prior to thyroidectomy, to find significant correlates of distress in the early cancer diagnosis phase.
Between June 2010 and March 2011, among 185 patients who were newly diagnosed with well-differentiated thyroid cancer and awaiting thyroidectomy at the Thyroid Cancer Center of the Yonsei University Gangnam Severance Hospital, 161 patients agreed to participate in this study. Overall refusal rate was 12.9%. Nine patients were additionally excluded due to excessive amounts of missing data on self-report form questionnaires. Finally, 152 patients of the ages of 19-69 years old were included in the final analysis.
Written informed consent was obtained from all participants. This study was approved by the Institutional Review Board of the Yonsei University Gangnam Severance Hospital.
Sociodemographic data including age, sex, education, occupational status, marital status, and family income were obtained using self-report questionnaires. Clinical characteristics related to cancer including stage, type, interval between diagnosis and operation, and thyroid function test scores were collected.
Regarding psychological distress, patient global distress levels were assessed using the Distress Thermometer (DT).19,20 DT was developed to efficiently monitor cancer-related distress18 and is included in many practice guidelines for the psychosocial care of patients all over the world including Korea.21 A recent meta-analysis suggested that this tool is an efficient screening method for cancer-related distress.22 A cut-off score of 4 on the DT was selected based on previous studies of distress groups.20,23 All of the patients were evaluated for their distress prior to undergoing a thyroidectomy.
Each patient's mental coping strategies were evaluated with the Korean version of the mini-Mental Adjustment to Cancer (K-MAC) scale.24 Watson, et al.25 colleagues developed this disease-specific questionnaire to evaluate mental adjustment to and coping with cancer called the MAC scale which evaluates five factors: helplessness-hopelessness, anxious-preoccupation, cognitive-avoidance, fatalism and fighting-spirit.
After a descriptive analysis of data in study subjects, independent t-test and chi-square analyses were conducted to compare sociodemographic and clinical characteristics between the distress and no-distress groups. Linear regression analysis was applied to evaluate the association of K-MAC scale factor scores, as well as demographic and clinical variables, with distress in thyroid cancer patients. The statistical threshold for significant results was 0.05 in two-sided probability.
The mean age of the patient group in this study was 43.1 years old and about three fourths of the subjects were female. The average education level was 14.3 years and 83.6% of the subjects lived with a partner. All of the patients were diagnosed with papillary thyroid cancer and TNM stage I was most common among them. The mean interval between diagnosis and operation was 67.1±2.9 days (mean±standard error). Their K-MAC scale factor scores showed no significant differences from the original standardization study.24 As shown in Table 1, there were no differences in sociodemographic characteristics including age (t=0.194, p=0.846), sex (chi-square=1.535, p=0.215), education years (t=-0.941, p=0.348), marital status (chi-square=4.189, p=0.123), occupation (chi-square=4.744, p=0.192), and family income status (chi-square=2.319, p=0.509) or clinical characteristics including cancer TNM stage (chi-square=2.213, p=0.331), cancer type (chi-square=2.032, p=0.154), waiting interval (t=-1.113, p=0.268), baseline thyroid function (T3: t=0.403, p=0.687; free T4: t=0.182, p=0.856; thyroid-stimulating hormone: t=0.022, p=0.983) between the distress and no-distress groups, except for helplessness-hopelessness (t=3.033, p=0.003) and anxious preoccupation factor (t=5.485, p<0.001) scores on the MAC scale.
The results of the regression analysis for distress among thyroid cancer patients are summarized in Table 2. In the present study, the linear regression model was significant, explaining 38.5% of total variance in distress of thyroid cancer patients. Distress level was significantly associated with the anxious-preoccupation factor and the helplessness-hopelessness factor on the MAC scale. Other demographic and clinical variables showed no significant association with distress in thyroid cancer patients awaiting thyroidectomy.
In the present study, negative mental adjustment including anxious-preoccupation and helplessness-hopelessness factor on the MAC scale was significantly associated with distress in thyroid cancer patients. When considering that survivors of thyroid cancer reported deteriorated QoL after cancer treatment7-9 and that emotional distress was the major determinant of poor QoL,10 decreasing distress in the early cancer treatment phase may be very important for preserving QoL in patients with thyroid cancer. We found that DT was a very useful and effective tool in screening for cancer-related distress in thyroid cancer patients in the initial phase of cancer treatment. In the present study, about a third of all thyroid cancer patients awaiting thyroidectomy (n=56, 28.9%) reported a significant distress level over 4 points on the DT. This result was consistent with a previous finding that 28.8% of Korean cancer patients reported significant psychological distress.26
Mental coping strategies for cancer are important modulating factors in psychological distress.13 In the present study, negative mental adjustment, reflected as helplessness-hopelessness and anxious-preoccupation factors on the MAC scale, were significantly associated with distress, but fighting-spirit and fatalistic-acceptance factors, which were identified as positive mental adjustment factors in a previous study for standardization of the K-MAC scale, exhibited no significant association with distress.19 The results of this study were in line with a previous study, in which a correlation between anxious-preoccupation with depressive symptoms in breast cancer patients was reported.27 Watson, et al.15 reported a maintaining modulation effect of baseline helplessness-hopelessness after 10-year follow-up of disease-free survivors of breast cancer patients. However, patients with high fighting spirit demonstrated no significant survival advantage,15 even though other previous studies with a smaller sample size reported a survival advantage associated with high fighting spirit.28,29 In their study, a high score for baseline depressive symptoms in breast cancer patients was associated with a significant reduction in the 5-year survival rate.30 However, it did not maintain statistical significance in the subsequent 10-year follow-up study.15 When breast cancer was diagnosed, the helplessness-hopelessness factor may be the most important psychological factor in maintaining a disease-free state after treatment. Self-efficacy is also known to contribute to emotional well-being31 and QoL32 in cancer patients, and MAC factors, including fighting-spirit, anxious-preoccupation, and helplessness-hopelessness, reportedly partially mediate self-efficacy.14
Although questions about the effects of psychosocial intervention on prolongation of cancer survival remains unresolved,33 there is sufficient evidence for the effect of psychosocial intervention on the improvement of QoL in cancer patients, especially for those experiencing significant psychological distress or emotional symptoms.34 Recent meta-analyses reported no significant effect of psychosocial intervention on overall survival rate,33,35 but a significant effect on the improvement of QoL in cancer patients.36
Cancer diagnosis may impose a psychological burden on cancer patients. However, some cancer patients may enhance their mental health after cancer treatment if appropriate psychological support is provided. In a long-term follow-up study conducted with Swedish adolescent cancer patients, the cancer group reported lower levels of mental health and vitality until 6 months after diagnosis, but reported significantly decreased levels of emotional distress and a higher level of vitality 18 months and 48 months after diagnosis than the control group. The authors of this study emphasized the importance of psychological support for patients experiencing distress and low QoL.37
Even though a previous study reported that female sex, low educational level and poor performance status were associated with distress in Korean cancer patients,26 demographic and clinical characteristics including gender, age, education, TNM stage and thyroid function showed no significant correlation with distress in thyroid cancer patients in the present study. Positive expectations concerning cancer prognosis may contribute to this contradictory finding because we included patients with well-differentiated thyroid cancer; however, the previous study included various kinds of newly diagnosed cancer patients. Thus, the results of this study cannot be generalized to patients of various kinds of cancer.
The present study was subject to several limitations. First, this is a cross-sectional study, and we did not provide patients with a psychosocial intervention program or follow them after surgical treatment. Accordingly, we did not observe the association of mental adjustment and psychological distress with treatment outcome and psychological changes in these patients. Second, we did not perform a structured interview for psychiatric diagnosis of the patients who reported significant psychological distress and emotional symptoms. We considered that most patients with significant distress in this study displayed a spectrum of anxiety, depression and adjustment disorder, which are the most common psychiatric disorders in cancer patients.38 Third, we could not investigate the relationship of distress with QoL because we did not assess QoL in cancer patients with a questionnaire. Since we assessed patients who were waiting for surgical operation in the present study, we tried to reduce the number of questions to as many as possible to avoid imposing additional stress of too many scaled items. Thus, we focused on the relationship between mental adjustment and distress.
In summary, negative mental adjustment including anxious-preoccupation and helplessness-hopelessness was associated with increased distress, while positive mental adjustment such as fighting-spirit showed no significant association with distress in the present study. Considering the possibility of psychological maturation toward a positive aspect with psychological support after cancer diagnosis and treatment, patients who are showing helplessness-hopelessness and anxious adjustment should be identified and provided appropriate psychiatric intervention after cancer diagnosis. Future studies are needed to investigate the effect of psychiatric intervention on thyroid cancer patients with negative mental adjustment and significant distress.
Figures and Tables
Table 1
Comparison of Sociodemographic and Clinical Characteristics According to Distress
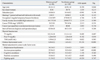
TSH, thyroid-stimulating hormone.
Values are presented as mean±standard error.
*The distress group is defined as a group of patients who rated their stress over 4 on the distress thermometer scale.
†Fisher's exact test was performed for distribution analysis of marriage, family income and cancer stage.
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant from Yonsei University College of Medicine for 2010 (6-2010-0140). The authors would like to acknowledge the assistance of Ms. Jee-Hee Oh in the collecting and management of research data.
Notes
References
1. Brown RL, de Souza JA, Cohen EE. Thyroid cancer: burden of illness and management of disease. J Cancer. 2011. 2:193–199.

2. Leenhardt L, Grosclaude P. [Epidemiology of thyroid carcinoma over the world]. Ann Endocrinol (Paris). 2011. 72:136–148.
3. Rometo DA, Baranski TJ. Thyroid cancer: what to do after fine needle aspiration. Mo Med. 2011. 108:93–98.
4. National Cancer Center. Cancer Facts and Figures 2011 (in Korean). 2011. Gyeonggi-do, Korea: Ministry of Health and Welfare;(http://ncc.re.kr/english/cyber/publi01.jsp).
5. Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992-2006. Thyroid. 2011. 21:125–134.

6. Thomas BC, Bultz BD. The future in psychosocial oncology: screening for emotional distress--the sixth vital sign. Future Oncol. 2008. 4:779–784.

7. Hoftijzer HC, Heemstra KA, Corssmit EP, van der Klaauw AA, Romijn JA, Smit JW. Quality of life in cured patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2008. 93:200–203.

8. Schultz PN, Stava C, Vassilopoulou-Sellin R. Health profiles and quality of life of 518 survivors of thyroid cancer. Head Neck. 2003. 25:349–356.

9. Taïeb D, Sebag F, Cherenko M, Baumstarck-Barrau K, Fortanier C, Farman-Ara B, et al. Quality of life changes and clinical outcomes in thyroid cancer patients undergoing radioiodine remnant ablation (RRA) with recombinant human TSH (rhTSH): a randomized controlled study. Clin Endocrinol (Oxf). 2009. 71:115–123.

10. Lee JI, Kim SH, Tan AH, Kim HK, Jang HW, Hur KY, et al. Decreased health-related quality of life in disease-free survivors of differentiated thyroid cancer in Korea. Health Qual Life Outcomes. 2010. 8:101.

11. Ayres A, Hoon PW, Franzoni JB, Matheny KB, Cotanch PH, Takayanagi S. Influence of mood and adjustment to cancer on compliance with chemotherapy among breast cancer patients. J Psychosom Res. 1994. 38:393–402.

12. Costanzo ES, Lutgendorf SK, Rothrock NE, Anderson B. Coping and quality of life among women extensively treated for gynecologic cancer. Psychooncology. 2006. 15:132–142.

13. Greer S, Moorey S, Watson M. Patient's adjustment to cancer: the Mental Adjustment to Cancer (MAC) scale vs clinical ratings. J Psychosom Res. 1989. 33:373–377.

14. Rottmann N, Dalton SO, Christensen J, Frederiksen K, Johansen C. Self-efficacy, adjustment style and well-being in breast cancer patients: a longitudinal study. Qual Life Res. 2010. 19:827–836.

15. Watson M, Homewood J, Haviland J, Bliss JM. Influence of psychological response on breast cancer survival: 10-year follow-up of a population-based cohort. Eur J Cancer. 2005. 41:1710–1714.

16. Tavoli A, Mohagheghi MA, Montazeri A, Roshan R, Tavoli Z, Omidvari S. Anxiety and depression in patients with gastrointestinal cancer: does knowledge of cancer diagnosis matter? BMC Gastroenterol. 2007. 7:28.

17. Dagan T, Bedrin L, Horowitz Z, Chaushu G, Wolf M, Kronenberg J, et al. Quality of life of well-differentiated thyroid carcinoma patients. J Laryngol Otol. 2004. 118:537–542.

18. Pelttari H, Sintonen H, Schalin-Jäntti C, Välimäki MJ. Health-related quality of life in long-term follow-up of patients with cured TNM Stage I or II differentiated thyroid carcinoma. Clin Endocrinol (Oxf). 2009. 70:493–497.

19. Roth AJ, Kornblith AB, Batel-Copel L, Peabody E, Scher HI, Holland JC. Rapid screening for psychologic distress in men with prostate carcinoma: a pilot study. Cancer. 1998. 82:1904–1908.

20. Shim EJ, Shin YW, Jeon HJ, Hahm BJ. Distress and its correlates in Korean cancer patients: pilot use of the distress thermometer and the problem list. Psychooncology. 2008. 17:548–555.

21. Yu ES, Shim EJ, Kim HK, Hahm BJ, Park JH, Kim JH. Development of guidelines for distress management in Korean cancer patients. Psychooncology. 2012. 21:541–549.

22. Mitchell AJ. Short screening tools for cancer-related distress: a review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2010. 8:487–494.

23. Wang GL, Hsu SH, Feng AC, Chiu CY, Shen JF, Lin YJ, et al. The HADS and the DT for screening psychosocial distress of cancer patients in Taiwan. Psychooncology. 2011. 20:639–646.

24. Kang JI, Chung HC, Kim SJ, Choi HJ, Ahn JB, Jeung HC, et al. Standardization of the Korean version of Mini-Mental Adjustment to Cancer (K-Mini-MAC) scale: factor structure, reliability and validity. Psychooncology. 2008. 17:592–597.

25. Watson M, Greer S, Young J, Inayat Q, Burgess C, Robertson B. Development of a questionnaire measure of adjustment to cancer: the MAC scale. Psychol Med. 1988. 18:203–209.

26. Kim SJ, Rha SY, Song SK, Namkoong K, Chung HC, Yoon SH, et al. Prevalence and associated factors of psychological distress among Korean cancer patients. Gen Hosp Psychiatry. 2011. 33:246–252.

27. Seok JH, Kim LS, Hong N, Hong HJ, Kim SJ, Kang HJ, et al. Psychological and neuroendocrinological characteristics associated with depressive symptoms in breast cancer patients at the initial cancer diagnosis. Gen Hosp Psychiatry. 2010. 32:503–508.

28. Greer S, Morris T, Pettingale KW. Psychological response to breast cancer: effect on outcome. Lancet. 1979. 2:785–787.

29. Pettingale KW, Morris T, Greer S, Haybittle JL. Mental attitudes to cancer: an additional prognostic factor. Lancet. 1985. 1:750.

30. Watson M, Haviland JS, Greer S, Davidson J, Bliss JM. Influence of psychological response on survival in breast cancer: a population-based cohort study. Lancet. 1999. 354:1331–1336.

31. Boehmer S, Luszczynska A, Schwarzer R. Coping and quality of life after tumor surgery: personal and social resources promote different domains of quality of life. Anxiety Stress Coping. 2007. 20:61–75.

32. Northouse LL, Mood D, Kershaw T, Schafenacker A, Mellon S, Walker J, et al. Quality of life of women with recurrent breast cancer and their family members. J Clin Oncol. 2002. 20:4050–4064.

33. Smedslund G, Ringdal GI. Meta-analysis of the effects of psychosocial interventions on survival time in cancer patients. J Psychosom Res. 2004. 57:123–131.

34. Spiegel D. Mind matters -- group therapy and survival in breast cancer. N Engl J Med. 2001. 345:1767–1768.

35. Chow E, Tsao MN, Harth T. Does psychosocial intervention improve survival in cancer? A meta-analysis. Palliat Med. 2004. 18:25–31.

36. Rehse B, Pukrop R. Effects of psychosocial interventions on quality of life in adult cancer patients: meta analysis of 37 published controlled outcome studies. Patient Educ Couns. 2003. 50:179–186.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download