Abstract
Purpose
To investigate the use of pretreatment carbohydrate antigen (CA) 19-9 and carcinoembryonic antigen (CEA) as prognostic factors to determine survival in pancreatic adenocarcinoma.
Materials and Methods
A retrospective review of the medical records of patients who were diagnosed with pancreatic adenocarcinoma and received surgery, chemoradiotherapy or chemotherapy was performed. Factors, including CA 19-9 and CEA, associated with the survival of pancreatic cancer patients were analyzed.
Results
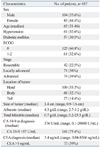
Patients with the median age of 65 years were included (n=187). Elevated serum CA 19-9 levels and CEA levels were observed in 75.4% and 39% of patients at diagnosis, respectively. CEA was correlated with tumor stages (p=0.005), but CA 19-9 was not. CA 19-9 and CEA were elevated in 69.0% and 33.3% of patients with resectable pancreatic cancer, and elevated in 72.9% and 47.2% of patients with advanced pancreatic cancer, respectively. The median overall survival of the normal serum CEA group was longer than that of the elevated serum CEA group (16.3 months vs. 10.2 months, p=0.004). However, the median overall survival of the normal serum CA 19-9 group was not different from that of the elevated serum CA 19-9 group (12.4 months vs. 13.5 months, p=0.969). The independent factors associated with overall survival were advanced pancreatic cancer [harzard ratio (HR) 4.33, p=0.001] and elevated serum CEA level (HR 1.52, p=0.032).
Pancreatic cancer is the fourth leading cause of cancer deaths in the United States. In 2011, 44030 patients were estimated to be diagnosed with pancreatic cancer, with an estimated 37660 deaths due to the disease.1 Surgical resection is the only curative treatment for pancreatic cancer. However, only 5-25% of patients with pancreatic cancer are candidates for curative pancreatectomy.2 Most of these patients are inoperable at diagnosis, and with other treatment modalities, the overall 5-year survival is less than 5%.3 Survival of pancreatic cancer patients has remained the similar since the development of Whipple's operation and gemcitabine. To provide more effective treatment to these patients, studies have set out to find biomarkers for better diagnosis and prognosis.
Prognostic factors can predict treatment response and by doing so assess the risk of disease progression. In the future, these markers may be able to guide personalized therapy. So far, several tumor markers of pancreatic cancer have been reported. Among them, carbohydrate antigen (CA) 19-9 is the standard tumor marker for pancreatic cancer. CA 19-9 has proven to be useful in differentiating benign from malignant pancreatic diseases. The sensitivity of CA 19-9 ranges from 41 to 86% with a specificity of 33 to 100%.4 Carcinoembryonic antigen (CEA) is the most commonly used tumor marker for gastrointestinal malignancies. It was originally developed for pancreatic cancer and was used throughout 1970-1980 before the advance of CA 19-9. Currently, CEA is the standard tumor marker for screening and predicting the prognosis of colorectal cancer.5
The possibility of utilizing both markers as prognostic factors has been tested in several studies. Kim, et al.6 reported that preoperative CA 19-9 levels could predict the resectability of pancreatic cancer. Also, pretreatment CA 19-9 level was proven to be a prognostic factor in pancreatic cancer treated with chemotherapy or chemoradiotherapy.7-9 Although the usefulness for CA 19-9 as a prognostic marker was reported, only specific tumor stages or cases treated with a specific treatment modality such as operation, chemotherapy or chemoradiotherapy was included in these previous studies. Considering its background and usefulness in gastrointestinal malignancies, CEA might be also useful in predicting pancreatic cancer, but less is known about the association between pretreatment CEA level and the prognosis of pancreatic cancer. The potential of CA 19-9 and CEA as prognostic factors has not been yet determined.
Thus, in this study we included patients with pancreatic cancer, regardless of stages and treatment modality, and analyzed the factors associated with survival to determine the utility of pretreatment CA 19-9 and CEA in assessing the prognosis of patients with pancreatic adenocarcinoma.
We reviewed the medical records of patients diagnosed with pancreatic cancer at Severance Hospital (Seoul, Korea) from August 2007 to December 2010. All patients were histologically diagnosed with pancreatic adenocarcinoma and underwent dynamic computed tomography (CT) of the abdomen and pelvis. The levels of CA 19-9 and CEA were evaluated before treatment. All patients received either an operation, chemotherapy or chemoradiotherapy (CRT), and patients who only received supportive care, palliative surgery or other treatments were not included in the study. The patients who were referred from other hospitals after receiving treatment or who refused treatment were also excluded. Also, patients with a history of other malignancies were excluded.
The clinical variables used in this study were sex, age, hypertension, diabetes mellitus, Eastern Cooperative Oncology Group (ECOG), stage, location of tumor, size of tumor, albumin, total bilirubin, CA 19-9 level, CEA level and treatment modality. The standard diagnostic cutoff values for CA 19-9 and CEA were used-37 U/mL and 5 ng/mL, respectively. CA 19-9 and CEA were measured using chemiluminescence immunoassay on the VITROS 3600 Immunodiagnostic System (Ortho-Clinical Diagnostics Inc., Raritan, NJ, USA) and the UniCel DxI 800 Access Immunoassay System (Beckman Coulter Inc., Brea, CA, USA), respectively. All tumors were classified as resectable pancreatic cancer (including stage I and II), locally advanced pancreatic cancer (including stage III) and advanced pancreatic cancer (including stage IV) using the American Joint Committee on Cancer (AJCC, the 7th edition) TNM staging system. TNM staging was based on CT scan or pathological results, if available. The Institutional Review Board approved this study for human research at Yonsei University College of Medicine.
Relationships between categorical variables were compared using χ2 test and comparisons of continuous variables in two groups were performed using Student's t-test. The correlations of CA 19-9 level and CEA level with tumor stages were evaluated using Spearman correlation. Survival in different subgroups was estimated by the Kaplan-Meier methods. The influence of potential prognostic factors on survival was assessed by multivariate analysis with the Cox proportional hazards model. Values of p<0.05 were considered statistically significant for all statistical analyses. Statistical analysis was performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA).
One hundred and eighty-seven patients were included in this study, and 104 (55.6%) patients were male. The median age was 65 years (range, 31-86 years). The initial ECOG scores were 0 in 125 patients (66.8%) and 1-2 in 61 patients (32.6%). Fourty-two patients (22.5%) were classified with resectable pancreatic cancer, 71 patients (38%) with locally advanced pancreatic cancer and 74 patients (39.6%) with advanced pancreatic cancer. Tumors were primarily located at the pancreas head (53.5%). The median size of the tumors was 3.4 cm (range, 0.9-7.6 cm). Nine patients (4.8%) underwent operation, 77 patients (41.2%) received chemotherapy and 101 patients (54%) were treated with CRT (Table 1). The regimens of chemotherapy included gemcitabine only in 19 patients (24.6%), gemcitabine plus capecitabine in 16 patients (20.7%), gemcitabine plus cisplatin in 23 patients (30.1%) and gemcitabine plus erlotinib in 19 patients (24.6%). Among the patients who received CRT, 82 patients (81.1%) underwent gemcitabine based CRT and 19 patients (18.9%) underwent 5-fluorouracil based CRT.
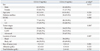
CA 19-9 and CEA were evaluated at initial diagnosis, and the median levels were 376 U/mL (range, 0.1-20000 U/mL) and 3.4 ng/mL (range, 0.04-8566 ng/mL), respectively. CA 19-9 was increased above 37 U/mL in 141 patients (75.4%) and CEA was increased above 5 ng/mL in 73 patients (39%).
In the elevated CA 19-9 level group, ECOG was higher (87.1% vs. 12.9%, p=0.009) and total bilirubin increased more (2.9±4.6 g/dL vs. 1.5±2.6 g/dL, p=0.009), compared to the normal CA 19-9 level group (Table 2). There was no significant difference in the size of tumors according to CA 19-9. In the elevated CEA level group, tumor location and tumor size were significantly different from the normal CEA level group. The sizes of tumors were larger compared to the normal CEA level group (3.8±1.4 cm vs. 3.2±1.0 cm, p=0.001). In the elevated CEA level group tumors were located mostly at the pancreas body and tail (49.4%), but primarily at the head (70%) in the normal CEA level group (p=0.007) (Table 3). There was no association between CA 19-9 level and tumor stages (p=0.150), but CEA increased as tumor stages progressed (p=0.005).
The comparison of survival according to serum CA 19-9 and CEA in patients with pancreatic cancer. The median follow-up period of all the patients was 11.7 months (range, 2-59.5 months). In total, 135 patients (72.2%) died by the time of the final analysis. The median overall survival of the normal serum CA 19-9 group and the elevated serum CA 19-9 were 12.4 months (range, 9.3-15.6 months) and 13.5 months (12.0-15.0 months), respectively (p=0.969) (Fig. 1A). The median overall survival of the normal serum CEA group and the elevated serum CEA group were 16.1 months (range, 12.2-19.9 months) and 10.2 months (range, 7.5-12.9 months), respectively (p=0.005) (Fig. 1B).
Unresectable patients were analysed to compare overall survival and progression free survival. There were 145 patients including locally advanced pancreatic cancer and advanced pancreatic cancer. There were 112 patients (77.2%) with elevated serum CA 19-9 and 59 patients (40.7%) with elevated CEA. The median overall survival of the normal CA 19-9 group and the elevated CA 19-9 group were 8.4 months and 11.6 months, respectively (p=0.597). However, the median overall survival between the normal CEA group and elevated CEA group were significantly different (13.4 months vs. 8.2 months, p=0.003).
In unresectable patients, the median progression free survival of the normal CA 19-9 group and elevated CA 19-9 group were 4.6 months and 5.3 months, respectively (p=0.735). The median progression free survival of the normal CEA group and elevated CEA group were 6.3 months and 3.7 months, respectively (p=0.012). The number of patients with elevated CEA level in resectable group was too small, the subgroup analysis was not done.
The association between survival and the parameters of sex, age, ECOG, diabetes mellitus, stage, location of tumor, size of tumor, level of CA 19-9 and CEA were analyzed by univariate analysis, which showed that ECOG (1 and 2), tumor stage (advanced stage), location of tumor (body & tail), size of tumor (>3 cm) and CEA (>5 ng/mL) were significantly associated with poor overall survival. In multivariate analysis, advanced pancreatic cancer stage [compared with resectable, harzard ratio (HR) 4.33, confidence interval (CI) 2.30-8.15, p=0.001] and CEA (>5 ng/mL) (HR 1.52, CI 1.03-2.23, p=0.032) were significant prognostic factors associated with overall survival (Table 4). Subgroup analysis in the unresectable group showed the same result. The advanced cancer stage (HR 2.46, CI 1.59-3.80, p=0.001) and CEA (>5 ng/mL) (HR 1.61, CI 1.07-2.42, p=0.022) were two independent prognostic factors affecting the overall survival of the unresectable group.
Pancreatic adenocarcinoma is an aggressive tumor with a poor prognosis. In addition, patients with pancreatic cancer are often diagnosed with metastatic disease or are at an inoperable status. Even though treatment plans are devised based on the stage of the tumor, the patient's condition, and several other clinical factors, not all patients benefit from conventional anti-cancer treatment. Studies have evaluated the efficacy of various tumor markers for improved prediction of treatment responses, risk of cancer progression, and medical costs in pancreatic cancer. The most widely used tumor markers for pancreatic cancer are CA 19-9 and CEA.
CA 19-9 was first isolated from a colorectal cell line and has since become the most widely used biomarker for pancreatic cancer.10 Although CA 19-9 is not suitable as a screening marker in asymptomatic patients, it is useful for differentiating benign disease from malignant pancreatic disease. Also, a few studies reported that preoperative CA 19-9 was correlated with resectability and prognosis after surgery.11,12 In addition, postoperative CA 19-9 can predict overall survival and disease-free survival after pancreatic cancer resection and adjuvant chemotherapy.13 CEA was found more than 45 years ago and has been primarily used to monitor colorectal cancer.14 CEA has also been used in pancreatic cancer, but its sensitivity and specificity are too low to be used as a diagnostic biomarker. Instead, a preoperative combination of CEA and CA 19-9 has been used to predict the resectability of pancreatic cancer.6,12,15 Moreover, a few studies suggested that pretreatment CEA was associated with poor treatment outcomes.16,17 However, these studies included a small number of patients, a specific tumor stage, a specific treatment modality, or applied a wide range of cutoff values. To determine whether CA 19-9 and CEA can be generally applicable prognostic markers of pancreatic cancer, the use of these biomarkers should be tested in a large number of patients with various stages of pancreatic cancer.
In this study, we analyzed 187 patients diagnosed with pancreatic adenocarcinoma. Cancers of stage 1 to 4 according to AJCC staging were all included and the standard diagnostic cutoff values of CA 19-9 and CEA were used. All patients received either an operation, chemotherapy, or CRT. In the elevated CEA level group, tumor size was larger than that of the normal CEA level group, and CEA level showed a positive correlation with tumor stages. In addition, our results showed that pretreatment CEA level was significantly associated with overall survival regardless of stages. In the unresectable group, the normal serum CEA level group showed longer progression free survival than the elevated serum CEA level group. However, the elevated CA 19-9 was not significantly associated with poor overall survival and progression free survival. There might be several reasons for this result. First, patients with Lewis blood type negative do not express the CA 19-9 antigen18 and inflammatory lesions of the pancreas can increase CA 19-9 level, even in low stages.19 Also obstructive jaundice might increase the level of CA 19-9, which is an important source of false positive results.20,21
Association between CEA and colon cancer is well known, but CEA has also been reported as a prognostic marker in variety of other cancers such as breast cancer, cervix cancer and lung cancer.22-24 In our study, CEA proved to be a potential prognostic marker of pancreatic cancer, especially in those treated non-surgically. In the surgically treated group, we failed to prove the usefulness of CEA because only a limited number of the patients with elevated CEA had resectable cancer. In the non-surgical group, CEA was not associated with the treatment response, but rather with progression free survival. This suggests that there was an acquisition of chemo-resistance in earlier periods and possibly different cancer behaviors. A few other studies showed an association between CEA and metastasis ability. CEA is expressed on the cell surface and functions in cellular adhesion.25 Therefore, malignant cells may aggravate and metastasize with increased CEA expressions. Recently, several cancer vaccines targeting CEA have been developed and they may improve treatment outcomes in pancreatic cancer patients with elevated CEA.26,27 From this point of view, pancreatic cancer is also a good target for cancer vaccines.
In conclusion, pretreatment elevated CEA level using the standard diagnostic cutoff-value contributed significant prognostic information on pancreatic cancer patients. Further studies are needed to establish whether CEA has a predictive value in regards to treatment modalities and chemotherapy regimens. Other potential biomarkers that could be useful for screening, diagnosing, and predicting treatment responses need to be further investigated and compared to CA 19-9 and CEA.
Figures and Tables
Fig. 1
Overall survival. (A) Comparison of survival time between the normal CA 19-9 group and the elevated CA 19-9 group. (B) Comparison of survival time between the normal CEA group and the elevated CEA group. CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen.

References
1. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011. 61:212–236.
2. Winek T, Hamre D, Mozell E, Vetto RM. Prognostic factors for survival after pancreaticoduodenectomy for malignant disease. Am J Surg. 1990. 159:454–456.

4. Bünger S, Laubert T, Roblick UJ, Habermann JK. Serum biomarkers for improved diagnostic of pancreatic cancer: a current overview. J Cancer Res Clin Oncol. 2011. 137:375–389.

5. Carriquiry LA, Piñeyro A. Should carcinoembryonic antigen be used in the management of patients with colorectal cancer? Dis Colon Rectum. 1999. 42:921–929.

6. Kim YC, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, et al. Can preoperative CA 19-9 and CEA levels predict the resectability of patients with pancreatic adenocarcinoma? J Gastroenterol Hepatol. 2009. 24:1869–1875.

7. Saad ED, Machado MC, Wajsbrot D, Abramoff R, Hoff PM, Tabacof J, et al. Pretreatment CA 19-9 level as a prognostic factor in patients with advanced pancreatic cancer treated with gemcitabine. Int J Gastrointest Cancer. 2002. 32:35–41.

8. Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008. 9:132–138.

9. Koom WS, Seong J, Kim YB, Pyun HO, Song SY. CA 19-9 as a predictor for response and survival in advanced pancreatic cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009. 73:1148–1154.

10. Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science. 1981. 212:53–55.

11. Halloran CM, Ghaneh P, Connor S, Sutton R, Neoptolemos JP, Raraty MG. Carbohydrate antigen 19.9 accurately selects patients for laparoscopic assessment to determine resectability of pancreatic malignancy. Br J Surg. 2008. 95:453–459.

12. Mehta J, Prabhu R, Eshpuniyani P, Kantharia C, Supe A. Evaluating the efficacy of tumor markers CA 19-9 and CEA to predict operability and survival in pancreatic malignancies. Trop Gastroenterol. 2010. 31:190–194.
13. Berger AC, Garcia M Jr, Hoffman JP, Regine WF, Abrams RA, Safran H, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008. 26:5918–5922.

14. Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965. 121:439–462.

15. Yasue M, Sakamoto J, Teramukai S, Morimoto T, Yasui K, Kuno N, et al. Prognostic values of preoperative and postoperative CEA and CA19.9 levels in pancreatic cancer. Pancreas. 1994. 9:735–740.

16. Kalser MH, Barkin JS, Redlhammer D, Heal A. Circulating carcinoembryonic antigen in pancreatic carcinoma. Cancer. 1978. 42:3 Suppl. 1468–1471.

17. Taylor OM, Cooper EH, Benson EA, McMahon MJ. The prognostic value of the tumour markers CA 195 and CEA in patients with adenocarcinoma of the pancreas. Eur J Surg Oncol. 1992. 18:508–513.
18. Tempero MA, Uchida E, Takasaki H, Burnett DA, Steplewski Z, Pour PM. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987. 47:5501–5503.
19. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007. 33:266–270.

20. Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, et al. The clinical value of serum CEA, CA 19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005. 31:164–169.

21. Peterli R, Meyer-Wyss B, Herzog U, Tondelli P. [CA 19-9 has no value as a tumor marker in obstructive jaundice]. Schweiz Med Wochenschr. 1999. 129:77–79.
22. Molina R, Augé JM, Escudero JM, Filella X, Zanon G, Pahisa J, et al. Evaluation of tumor markers (HER-2/neu oncoprotein, CEA, and CA 15.3) in patients with locoregional breast cancer: prognostic value. Tumour Biol. 2010. 31:171–180.

23. Borras G, Molina R, Xercavins J, Ballesta A, Iglesias J. Tumor antigens CA 19.9, CA 125, and CEA in carcinoma of the uterine cervix. Gynecol Oncol. 1995. 57:205–211.

24. Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012. 76:138–143.

25. Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989. 57:327–334.

26. Kaufman HL, Lenz HJ, Marshall J, Singh D, Garett C, Cripps C, et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res. 2008. 14:4843–4849.

27. Gulley JL, Madan RA, Tsang KY, Arlen PM, Camphausen K, Mohebtash M, et al. A pilot safety trial investigating a vector-based vaccine targeting carcinoembryonic antigen in combination with radiotherapy in patients with gastrointestinal malignancies metastatic to the liver. Expert Opin Biol Ther. 2011. 11:1409–1418.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download