Abstract
Purpose
S100B protein is widely used as a measure of glial activity or damage in several brain conditions. Central nervous system (CNS) infections can cause neurological sequelae because of parenchyma invasion. It is difficult to predict further neuronal damage in the CNS infection. The present study is aimed to evaluate the role of the cerebrospinal fluid (CSF) S100B protein as an indicator of neuronal damage in CNS infection.
Materials and Methods
We measured the concentration of CSF S100B protein in 62 patients with a CNS infection using an Enzyme-Linked Immunosorbent Assay. The patients with CNS infections were classified as having no neuronal damage (CNS-N) or as having neuronal damage (CNS+N) according to the presence of neurological change or structural lesions on brain MRI.
Results
The CSF S100B protein level of the CNS+N group (n=22, 0.235 µg/L, 0.10-2.18) was significantly higher than that of the CNS-N group (n=40, 0.087 µg/L, 0.06-0.12) and control group (n=40, 0.109 µg/L, 0.07-0.14, p<0.01). Using an arbitrary cut off value, S100B-positive CSF was detected in 2.5% of the CNS-N group and in 50% of the CNS+N group (p<0.05).
Central nervous system (CNS) infection is often associated with a fatal outcome or permanent damage, including cognitive and behavioral impairment, mental change, seizure, and focal neurological deficits such as hemiparesis, dysarthria, diplopia, and aphasia.1-6 Thus, early identification and proper treatment of neuronal damage are essential for optimal prognosis. However, it is currently difficult to make a prognosis, particularly in cases with substantial brain damage; thus, development of a reliable marker for neuronal damage may be of prognostic value for CNS infections.
S100B is a cytoplasmic calcium-binding protein that is abundant in astroglial cells in the CNS7 and regulates intracellular and extracellular calcium activity. Although the mechanism underlying S100B secretion is unknown, the protein has been shown to increase in the cerebrospinal fluid (CSF) and serum following various neurological insults, including traumatic head injury, subarachnoid hemorrhage, and cerebral infarction, and cardiac arrest.8-11
CSF concentrations of glial fibrillary acidic protein (GFAP) and neuron-specific enolase (NSE) have been used as markers of neuronal destruction. In contrast to GFAP and NSE, S100B release is independent of natural cell death and the status of the extracellular medium.12 The concentration of this protein in the CSF is comparable to that in brain tissue, suggesting that S100B concentrations indicate the degree of neuronal damage.12 Experimental evidence has shown that S100B is increased in the CSF of rabbits with pneumococcal meningitis.13 Elevated S100B protein levels in the CSF of infants with bacterial meningitis are related to the risk of brain lesions.14 Previous studies have demonstrated that serum S100B levels were higher in patients who had CNS infections than in patients with extracerebral infections.15 However, few studies have investigated the usefulness of CSF S100B protein as a specific marker for neuronal damage in adult patients with CNS infections.
The present study evaluated the association between acute-phase CSF S100B levels in patients with CNS infection and presence of neuronal damage.
Sixty-two patients from Uijeongbu St. Mary's Hospital, Catholic University of Korea, who had CNS infections confirmed by clinical diagnosis and CSF studies including culture, were consecutively enrolled in the present study. Bacterial/tuberculosis CNS infections were confirmed by the presence of pleocytosis in the CSF, a Gram-positive bacterial stain, an acid-fast bacillus stain, and tuberculous polymerase chain reaction or positive bacterial or tuberculous cultures. Viral CNS infections were confirmed as the presence of pleocytosis in a negative Gram stain and a bacterial/tuberculous culture. Patients were classified as having neuronal damage (CNS+N) or having no neuronal damage (CNS-N) at the time of discharge. Neuronal damage was diagnosed by mental change or focal neurological signs, the presence of seizure, abnormal electroencephalogram findings, and a magnetic resonance imaging (MRI) scan showing acute parenchymall lesions of the brain in more than leptomeninges. Patients diagnosed with acute benign headache (n=40) who had a normal neurological examination and normal results on computed tomography or MRI scans, and lumbar puncture, served as controls. All subjects who experienced stroke, brain tumor or head trauma within 3 months and who had previous neurological sequelae were excluded.
A person who was blind to the diagnosis of each patient performed measurement of CSF S100B concentrations. The CSF samples were stored at -20℃. S100B protein concentrations were analyzed using a commercially available Enzyme-Linked Immunosorbent Assay kit (BioVendor Laboratorni Medicina, A.S., Brno, Czech Republic.) according to the manufacturer's protocol. Duplicate 40 µg CSF samples were applied to well plates. After washing three times, biotin-labeled antibody solution was added and the samples were incubated for 60 minutes. After washing five times, the samples were incubated in Streptavidin-HRP conjugate for 30 minutes, and then the wells were washed five times. Then, substrate solution was added. The reaction was stopped by stop solution, and the absorbance was measured at 450 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
CSF S100B protein data that did not follow a normal distribution are expressed as median and interquatile range. Analysis of the CNS-infection subgroups was performed using the nonparametric Kruskal-Wallis test. When the result was significant, the Mann-Whitney U test was performed to make comparisons between subgroups.
Following the result of a pilot test, to detect the differences between 2 groups by t-test, the minimal required sample size was 98 under the assumption of alpha error 0.05, power 0.8 and calculated effect size d=0.615. Normal S100B protein levels in humans have not been determined and, because the cutoff values for the presence of neuronal damage are unknown, we defined positive values as three standard deviations above the mean values observed in the control group.
SPSS version 16 (SPSS Inc., Chicago, IL, USA) was used to conduct statistical tests. Two-tailed p values <0.05 were deemed statistically significant.
Sixty-two patients with a CNS infection were included in the study. Of those, 44 were diagnosed with a viral infection (34 viral meningitis and 10 viral encephalitis), six with a bacterial infection, and 12 patients were diagnosed with a tuberculous infection. Patient age ranged from 17-79 years (mean 38.1 years), and 55% were females. The CNS+N group comprised 22 patients (45.9±17 years, 41% female) and 40 patients were in the CNS-N group (33.9±13 years, 67% female). All CSF samples were obtained on the day of hospitalization to measure the changes of CSF S100B in acute phase. Duration from headache onset to CSF sample was 4.7 days.
The analysis of CSF S100B protein according to group revealed that S100B levels in the CNS+N group (0.235 µg/L, 0.10-2.18) were significantly higher than those in the CNS-N (0.087 µg/L, 0.06-0.12) and control groups (0.109 µg/L, 0.07-0.14; p<0.01). No significant difference in S100B protein levels was found between the CNS-N and control groups (p=0.147). The CSF was S100B positive in 2/40 (5%) of the CNS-N group and in 10/22 (45%) of the CNS+N group (p<0.01) (Fig. 1, Table 1).
The comparison among CNS infection subtypes revealed that S100B levels in patients with tuberculous infection tended to be higher than those in the other subgroups are; however, the difference between the patients with viral (0.101 µg/L; 0.05-0.15), bacterial (0.106 µg/L; 0.07-0.23), and tuberculous infections (0.127 µg/L; 0.11-1.09) was not statistically significant (p=0.143) (Fig. 2).
The present study demonstrated that CSF levels of S100B protein were significantly higher in patients with CNS infections who had neuronal damage than in those who did not have neuronal damage.
S100B protein is expressed by astrocytes, and at least 80% of the total S100B protein pool is found in the brain.7,16,17 S100B inhibits protein phosphorylation by interacting with kinase substrates and plays a role in assembling important components of cell cytoarchitecture.18,19 Little is known about the functional implications of S100B secretion by astrocytes, but a substantial amount of evidence indicates that S100B exerts trophic or toxic effects on neuronal cells depending on its concentration.19 At normal levels, S100B may have a neuroprotective role against glutamate toxicity.20 The protein stimulates neurite outgrowth21 and enhances survival of neurons after injury,22 and local administration of S100B has been reported to stimulate regeneration of injured rat sciatic nerve in vivo.23 In contrast, high levels of S100B resulting from an astrocytic reaction or reactive astrogliosis exacerbate neuroinflammation and neuronal damage.24 Recent observations have shown that high concentrations of S100B protein induce apoptotic death by interacting with the receptor for advanced glycation end product to elevate reactive oxygen species and activate the caspase cascade.25
S100B levels are elevated in a variety of CNS disorders.8-11 Although S100B release may be an effect of the condition rather than the cause, it is nonetheless highly associated with neuronal damage and, as such, is a strong candidate for a surrogate marker of CNS injury.19 Connolly, et al.26 reported a correlation between S100B concentrations and neurocognitive decline after carotid endarterectomy. A recent study of serum S100B protein in patients with systemic lupus erythematosis (SLE) reported that S100B levels were notably raised in SLE patients with neuropsychiatric manifestations and were related to the degree of neurological injury.27,28 CSF and serum S100B levels decreased and neuropsychiatric manifestations improved after 3 months in patients who accepted a standardized treatment, suggesting that the neuropsychiatric manifestations were induced by anti-neuronal antibodies, which ultimately caused neuron destruction and alterations in excitability.27 Elevated serum S100B protein levels have been found to be related to poor outcomes as measured by the Glasgow Coma Scale in patients with cerebral infectious disease.15 Furthermore, serum S100B protein levels have been shown to be associated with outcome in adults with ischemic stroke, post cardiac arrest, and post traumatic brain injury.8,29-31 We excluded patients with stroke, brain tumor or head trauma history within the past 3 months for the removal of the influences from other diseases.
In the present study, CSF S100B protein levels tended to be higher in patients with a tuberculous infection than in those with a viral infection, or the control group; however, the difference was not statistically significant. This may have been the result of the wide variation in S100B levels in patients with tuberculous infection. It is possible that tuberculous and bacterial infections, which had a high rate of accompanying neuronal damage, created bias toward higher levels of CSF S100B protein. Spinella, et al.32 analyzed CSF S100B protein in children with meningitis according to etiology and found that those with bacterial and tuberculous meningitis had elevated S100B protein levels compared with those who had aseptic meningitis; however, the difference failed to reach statistical significance. Undén, et al.15 reported that patients with bacterial meningitis tended to have higher levels of serum S100B protein than did patients with viral meningitis, but the difference was not statistically significant. Of particular importance, serum S100B levels were higher in the bacterial meningitis group than in patients with extracerebral bacterial infections such as bacterial pneumonia and bacterial enteritis, suggesting that an elevation in S100B protein levels is not dependent on etiology. Thus, the evaluation of CSF S100B is more likely to be related to the severity of neuronal damage than to the cause of the CNS infection.
To summarize, the results of the present study indicate that the high concentrations of S100B protein in the CSF associates with the neuronal damage after CNS infection. Further clinical or experimental studies are needed to identify the CSF S100B levels to predict the neuronal damage and to verify a causal relationship between neuronal damage and S100B protein in the pathogenesis of the CNS infections.
Figures and Tables
Fig. 1
The scatter distributions of CSF S100B levels in CNS-N, CNS+N, and control groups. Horizontal bars represent median values of each group. CSF, cerebrospinal fluid; CNS, central nervous system.
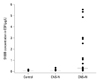
Fig. 2
Box plot of CSF S100B levels in the control group and in patients with viral, bacterial, and tuberculous CNS infections. The white line in the middle of the box represents median value. The box extends from the 25th to the 75th percentile. The whiskers extending from the box represent the 10th and 90th percentiles. Circles represent individual patient levels that extend above the 90th percentile. CSF, cerebrospinal fluid; CNS, central nervous system.

References
1. van de Beek D, Schmand B, de Gans J, Weisfelt M, Vaessen H, Dankert J, et al. Cognitive impairment in adults with good recovery after bacterial meningitis. J Infect Dis. 2002. 186:1047–1052.

2. Schmidt H, Heimann B, Djukic M, Mazurek C, Fels C, Wallesch CW, et al. Neuropsychological sequelae of bacterial and viral meningitis. Brain. 2006. 129(Pt 2):333–345.

3. Kim MA, Park KM, Kim SE, Oh MK. Acute symptomatic seizures in CNS infection. Eur J Neurol. 2008. 15:38–41.

4. Rufa A, Cerase A, Annunziata P, De Santi L, Buccoliero R, Monti L, et al. Transient supranuclear paresis of the abduction in viral encephalitis of the brainstem. Neurol Sci. 2010. 31:653–655.

5. de Louvois J, Halket S, Harvey D. Effect of meningitis in infancy on school-leaving examination results. Arch Dis Child. 2007. 92:959–962.

6. Bedford H, de Louvois J, Halket S, Peckham C, Hurley R, Harvey D. Meningitis in infancy in England and Wales: follow up at age 5 years. BMJ. 2001. 323:533–536.

7. Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001. 33:637–668.

8. Böttiger BW, Möbes S, Glätzer R, Bauer H, Gries A, Bärtsch P, et al. Astroglial protein S-100 is an early and sensitive marker of hypoxic brain damage and outcome after cardiac arrest in humans. Circulation. 2001. 103:2694–2698.

9. Ingebrigtsen T, Romner B, Marup-Jensen S, Dons M, Lundqvist C, Bellner J, et al. The clinical value of serum S-100 protein measurements in minor head injury: a Scandinavian multicentre study. Brain Inj. 2000. 14:1047–1055.

10. Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000. 31:2670–2677.

11. Wiesmann M, Missler U, Hagenström H, Gottmann D. S-100 protein plasma levels after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien). 1997. 139:1155–1160.

12. Goncalves CA, Leite MC, Nardin P. Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin Biochem. 2008. 41:755–763.

13. Schmidt H, Gerber J, Stuertz K, Djukic M, Bunkowski S, Fischer FR, et al. S100B in the cerebrospinal fluid--a marker for glial damage in the rabbit model of pneumococcal meningitis. Neurosci Lett. 2010. 475:104–107.

14. Gazzolo D, Grutzfeld D, Michetti F, Toesca A, Lituania M, Bruschettini M, et al. Increased S100B in cerebrospinal fluid of infants with bacterial meningitis: relationship to brain damage and routine cerebrospinal fluid findings. Clin Chem. 2004. 50:941–944.

15. Undén J, Christensson B, Bellner J, Alling C, Romner B. Serum S100B levels in patients with cerebral and extracerebral infectious disease. Scand J Infect Dis. 2004. 36:10–13.

16. Hidaka H, Endo T, Kawamoto S, Yamada E, Umekawa H, Tanabe K, et al. Purification and characterization of adipose tissue S-100b protein. J Biol Chem. 1983. 258:2705–2709.

17. Kindblom LG, Lodding P, Rosengren L, Baudier J, Haglid K. S-100 protein in melanocytic tumors. An immunohistochemical investigation of benign and malignant melanocytic tumors and metastases of malignant melanoma and a characterization of the antigen in comparison to human brain. Acta Pathol Microbiol Immunol Scand A. 1984. 92:219–230.
18. Pozdnyakov N, Margulis A, Sitaramayya A. Identification of effector binding sites on S100 beta: studies with guanylate cyclase and p80, a retinal phosphoprotein. Biochemistry. 1998. 37:10701–10708.

19. Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? J Neurosci Res. 2007. 85:1373–1380.

20. Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999. 1450:191–231.

21. Kligman D, Marshak DR. Purification and characterization of a neurite extension factor from bovine brain. Proc Natl Acad Sci U S A. 1985. 82:7136–7139.

22. Barger SW, Van Eldik LJ, Mattson MP. S100 beta protects hippocampal neurons from damage induced by glucose deprivation. Brain Res. 1995. 677:167–170.

23. Haglid KG, Yang Q, Hamberger A, Bergman S, Widerberg A, Danielsen N. S-100beta stimulates neurite outgrowth in the rat sciatic nerve grafted with acellular muscle transplants. Brain Res. 1997. 753:196–201.

24. Ahlemeyer B, Beier H, Semkova I, Schaper C, Krieglstein J. S-100beta protects cultured neurons against glutamate- and staurosporine-induced damage and is involved in the antiapoptotic action of the 5 HT(1A)-receptor agonist, Bay × 3702. Brain Res. 2000. 858:121–128.

25. Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000. 275:40096–40105.

26. Connolly ES Jr, Winfree CJ, Rampersad A, Sharma R, Mack WJ, Mocco J, et al. Serum S100B protein levels are correlated with subclinical neurocognitive declines after carotid endarterectomy. Neurosurgery. 2001. 49:1076–1082.

27. Yang XY, Lin J, Lu XY, Zhao XY. Expression of S100B protein levels in serum and cerebrospinal fluid with different forms of neuropsychiatric systemic lupus erythematosus. Clin Rheumatol. 2008. 27:353–357.

28. Schenatto CB, Xavier RM, Bredemeier M, Portela LV, Tort AB, Dedavid e Silva TL, et al. Raised serum S100B protein levels in neuropsychiatric lupus. Ann Rheum Dis. 2006. 65:829–831.

29. Spinella PC, Dominguez T, Drott HR, Huh J, McCormick L, Rajendra A, et al. S-100beta protein-serum levels in healthy children and its association with outcome in pediatric traumatic brain injury. Crit Care Med. 2003. 31:939–945.

30. Raabe A, Grolms C, Sorge O, Zimmermann M, Seifert V. Serum S-100B protein in severe head injury. Neurosurgery. 1999. 45:477–483.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download