Abstract
Purpose
There are no reports about bone graft and cell therapy for the osteonecrosis of femoral head (ONFH). We prospectively evaluated the clinical results of auto-iliac cancellous bone grafts combined with implantation of autologous bone marrow cells for ONFH.
Materials and Methods
Sixty-one hips in 52 patients with ONFH treated with bone graft and cell therapy were enrolled, and the average follow-up of the patients was 68 (60-88) months. Necrotic lesions were classified according to their size by the Steinberg method and location of necrosis.
Results
At the last follow-up, the percentage of excellent or good results was 80% (12/15 hips) in the small lesion group, 65% (17/26 hips) in the medium size group, and 28% (6/20 hips) in the large size group. The procedures were a clinical success in 4 of 5 hips (80%) of stage I, 23 of 35 hips (65.7%) of stage II, 7 of 18 hips (38.9%) of stage III, and 1 of 3 hips (33.3%) of stage IV grade, according to the Association Research Circulation Osseous grading system. Among the 20 cases with large sized necrotic lesions, 17 cases were laterally located and this group showed the worst outcomes, with 13 hips (76.5%) having bad or failed clinical results.
Conclusion
The results of the present study suggested that patients who have a large sized lesion or medium sized laterally located lesion would not be good candidates for the head preserving procedure. However, for medium sized lesions, this procedure generated clinical results comparable to those of other head preserving procedures.
The natural history of osteonecrosis of the femoral head (ONFH) is not clearly understood, but large sized ONFH often progresses till the hip is destroyed, necessitating total hip replacement arthroplasty.1-7 Although, numerous techniques have been introduced, there is no consensus regarding treatment algorithms. There are several conservative measures to preserve the femoral head, but no single therapeutic method has proven to be effective for prevention of disease progression.8-12 Steinberg, et al.13 reported that central decompression attained only a 64% success rate, and an autologous bone graft is advised to be performed simultaneously. The possibility has been raised that bone marrow containing osteogenic precursors implanted into a necrotic lesion of the femoral head may be of benefit in the treatment of this condition.13-19 Based on this theory, previous studies reported a 94% short-term success rate after autologous bone marrow mononuclear cell transplantations. Currently, there are no mid-term or long-term follow-up results of cell therapy for ONFH. As far as we know, there are no reports of bone graft and cell therapy for ONFH. Therefore, we performed a prospective clinical and radiological evaluation of ONFH treated with core decompression combined with autoiliac bone graft and implantation of autologous bone marrow cells.
Sixty-one hips (52 patients) diagnosed as ONFH and treated with autoiliac cancellous bone grafts combined with implantation of autologous bone marrow cells were enrolled in this study. A diagnosis of osteonecrosis was made on the basis of clinical history and the appearance of lesions on anterior-posterior, frog-leg lateral, and lateral plain radiographs, as well as magnetic resonance images. Simple X-rays were taken at follow-up every 3 months after the operation. Radiographs were also assessed for evidence of collapse of the head, measured by the distance between the superior subchondral border of the femoral head and the head-neck junction. For radiologic evaluation, we used several parameters, such as maintenance of the joint line, congruency of the femur head, and changes in bony trabeculae. This prospective clinical study was approved by the Institutional Review Board of Inha University Hospital.
Patient demographics and hospital data are summarized in Table 1. The average follow-up of the patients was 68 (60-88) months. No patients were lost to follow-up. The patients were followed up every 3 months for 1 year and annually thereafter. They were assessed both clinically and radiographically. Clinical evaluation was performed with the scoring system of Merle d'Aubigne and Postel. The Merle d'Aubigne and Postel hip score includes the parameters of pain, mobility, and ability to walk, each rated from 0 points (worst condition) to 6 points (best condition). Total scores exceeding 12 points are classified as excellent, 9 to 12 as good and 6 to 9 as bad. Scores lower than 6 points are regarded as a failure. Sixteen patients (30%) had a history of excessive alcohol intake, 6 (12%) patients reported the use of corticosteroids, 6 patients (12%) had experienced trauma (6 patients, 12%), and 22 patients (43%) exhibited no identifiable cause. The mean age of the patients at the time of the procedure was 44 years old (ranging from 19 to 66 years), There were 38 males (73%) and 14 females.
The classification of the size of femoral head osteonecrosis was based on the Steinberg method.20 Twenty hips were classified as large lesions (necrotic lesion >30%), 26 cases as medium sized lesions (15% to 30%) and 15 as small lesions (<15%). The size of the necrotic lesion was calculated by multiplying the percentage of the lesion seen in the antero-posterior view by that see in the lateral view using the magnified femoral head on picture archiving and communication system view on a section paper using hip AP, frog-leg lateral and lateral plain radiographs. When the necrotic border was not clear on simple X-ray, additional magnetic resonance imaging findings were recruited to measure the size of the necrotic lesion. According to the Association Research Circulation Osseous (ARCO) grading system, 5 hips were of stage I, 35 hips were of stage II, 18 hips were of stage III and 3 hips were of stage IV.
Bone marrow was aspirated from the anterior iliac crests with a 3 mm trocar and heparin coated 50 cc-syringes. Then, 110 mL of marrow was aspirated and diluted with the same amount of Hanks' Balanced salt solution (HBSS: GIBCO-BRL, Grand Island, NY, USA). Ficoll-Paque Plus (1077 g/L; Amersham Biosciences, Piscataway, NJ, USA) solution was added to the same ratio. A 30-minute centrifugation at 1000 G separated the mononuclear cells from the remainder of the marrow. The mononuclear cells were then collected and washed with HBSS 3 times before another 15-minute centrifugation at 900 G. Afterwards, the mononucleocyte layer was collected and 1.8 mL of phosphate buffered saline (Gibco, Carlsbad, CA, USA) was added to suspend the cells. The average density of the autologous marrow monocytes ranged from 16 to 52 million/mL, with a mean of 38±6 million/mL. The mean number of fibroblast colony forming units per one million nucleated cells obtained from each patient ranged from 18 to 73. After concentration, an average of 2.7×107 progenitor cells was obtained.
Patients were placed on the fracture table in the supine position. Healthy cancellous bone was obtained from the iliac crest and made into 10×5 mm sized bone chips for transplantation. Then, the approach to the necrotic head was made through a 4 cm sized lateral longitudinal incision at the area of the trochanter. Preoperative magnetic resonance images were used to identify the accurate site of the lesion, and with the aid of intraoperative image intensifiers, a guide pin was inserted from just distal to the greater trochanter to the site of the lesion. Core decompression was done by insertion of a 10 mm reamer along the guide pin, and the debridement of necrotic bone tissue was done using a reamer, a curette and Midas Rex. During this process we removed the necrotic bone as thoroughly as possible. Through the hole made with the reamer, the autoiliac cancellous bone chips were inserted and packed into the space in the femoral head for structural strength. Then, allogenous bone chips were packed into the tract of the femoral neck and the bone defect of the trochanteric area, which was made during core decompression. A new drill hole was made into the femoral head where the autologous cancellous bone graft was done, and a 20-gauge long spinal needle was inserted through the drill hole and the harvested autologous mesenchymal mononuclear cells were injected through the needle. Only partial weight bearing was allowed for 6 weeks after operation, after which full weight bearing was progressively allowed for another 6 weeks.
Data were assessed using Wilcoxon paired samples in order to compare the baseline data with the values obtained throughout the research. SPSS statistical software (version 11.0; SPSS, Chicago, IL, USA) was used to conduct the analyses. The data were considered significant at p-values of less than 0.05.
Overall, 15 hips (24.6%) had excellent clinical results, 20 hips (32.8%) demonstrated good clinical results and 26 hips (42.6%) showed bad or failed results. Through the procedures performed in this study, clinical success was observed in 4 of 5 hips (80%) of stage I, 23 of 35 hips (65.7%) of stage II, 7 of 18 hips (38.9%) of stage III and 1 of 3 hips (33.3%) of stage IV grading, according to the ARCO grading system.
Among 20 cases with preoperative severe necrotic lesions, 14 hips (72%) revealed bad or failed clinical results and 6 hips (28%) showed excellent and good results. Among 26 cases of moderate necrotic lesions, 17 cases (65%) presented excellent to good results, while the other 9 cases (35%) demonstrated bad or failed outcomes. Among 15 hips of mild necrotic lesions, a total of 3 hips (20%) resulted in bad or failed results, whereas the other 12 cases (80%) exhibited excellent or good results (Table 2). The operation time on average was 145.5 minutes and blood loss was 556 mL in severe necrotic lesions (Table 3). Among 4 hips out of 15 with mild necrotic lesions at a lateral location, 2 hips exhibited bad or failed outcomes (Table 4). Eighteen hips out of 26 presented with medium sized lesions at a lateral location, among which 9 hips (50%) showed bad or failed results (Table 4). Among the 20 cases with large sized necrotic lesions, 17 cases were laterally located and this group showed the worst outcomes, with 13 hips (76.5%) having bad or failed clinical results (Table 4). Thirteen hips showed collapse of the femoral head more than 2 mm, after a mean postoperative period of 7.6 months (range 3 to 20 months). In cases of excellent or good clinical results, the mean Merle d'Aubigne and Postel score improved from 11.6 points preoperatively to 15.5 points at the time of the final follow-up. Additionally, the mean pain score improved from 3.8 to 5.3 points. The mean mobility score improved from 3.8 to 5.1, and the mean walking ability score improved from 4.0 to 5.1.
The natural history of ONFH is not clear, but 60% of cases of femoral head osteonecrosis without symptoms eventually become symptomatic and 70 to 80% require total hip arthroplasty.1-6,21 The pathogenesis of ONFH is not clearly understood. Decreased femoral head blood flow can occur via three mechanisms: vascular interruption, extravascular compression and thrombotic occlusion. Intraosseous hypertension causes microvascular occlusion, resulting in nutrient artery occlusion, and finally accelerates the progression of osteonecrosis. Therefore, elevated pressure in the femoral head plays not only a critical role in early diagnosis of ONFN, but also in disease progression. Core decompression lessens pain by lowering the high pressure in the femoral head, and also regenerates the circulation compromised by such high pressure, thereby stimulating bone regeneration.22-26 However, structural regeneration after core decompression is incomplete. Koo, et al.9 claimed that core decompression causes only symptomatic relief of pain, but has no effect on femur head preservation. Thus, core decompression is usually combined with a variety of techniques (non-vascularized bone graft, vascularized fibular graft and electrical stimulation) for enhancing osteogenesis and bone repair.5,6,13,16 Mont, et al.11 suggested that performing a trapdoor procedure, which applies cancellous and cortical bone grafts together, after core decompression in ONFH patients is effective in preventing collapse of the femoral head by supporting the subchondral region structurally after removing osteonecrotic lesions.
Recently, a good amount of research is being published regarding the role of mesenchymal stem cells in ONFH. Hernigou, et al.17,18 reported that a decreased level of stem cell and stromal cell activity is found in femoral head osteonecrosis caused by alcohol and steroid consumption. Also, the activity of fibroblast colony forming units was decreased in ONFH with steroid use. This suggests that there is a decreased level of mesenchymal stem cell activity that leads to lower bone repair or redevelopment capability. Gangji, et al.15 reported that the proliferative rate of osteocytes sampled in the trochanteric region of femoral head osteonecrosis was lower than that of osteoarthritis, but the proliferative rate of long bone was similar to osteoarthritis. Hernigou and Beaujean17 additionally reported that a femoral head conservation rate of 94% was attained at 5- to 10-year follow-up by applying core decompression and autologous bone marrow transplantation in early femoral head osteonecrosis. Based on the data from the present study, we concluded that the most ideal method to treat the disease is by both removing as much necrotic bone as possible and applying bone graft with biological cellular treatment for structural support of the subchondral region. During stem cell implantation, some of the bone-marrow cells might have leaked out through the cannula or into the periphery of the proximal femur; however, we assume that the majority of the bone marrow cells remained in the osteonecrotic area or in the femoral head. This was shown by Gangji, et al.14 during his research by radionuclide labeling, which was performed in two patients. We were not able to find the exact location of the bone marrow cells after implantation using imaging techniques, thus larger trials and more advanced techniques may be needed to confirm the results.
Femoral head conservation methods should be relatively non invasive and lead to less severe complications, because they do not guarantee radical cure. From this point of view, the authors performed autologous bone graft and bone marrow transplantation. However, the overall clinical results of our procedure were not satisfactory. The femoral head conservation rate in ARCO stage II lesions was 64.7% and 37.0% in stage III lesions. These data are not consistent with previous reports of excellent results after cell therapy.14,16,19 The outcomes of auto-iliac cancellous bone grafting combined with implantation of autologous bone marrow cells after core decompression differed depending on the size of the lesion, and larger and more laterally located preoperative lesions demonstrated unfavorable outcomes. However, for medium sized lesions, this procedure may provide clinical results comparable to those of other head preserving procedures (Figs. 123-4).
A limitation to this study is that it was not a comparative study. Thus, we were unable to clarify the roles of each procedure (core decompression, bone graft and stem cell injection). Also, being unable to prove the mechanism of regeneration by stem cell implementation was another limitation.
Figures and Tables
Fig. 2
Initial MRI revealed bilateral medium sized ONFH. The patient complained of right hip pain, but none at the left hip. We administered a bone graft and cell therapy for the right hip only. MRI, magnetic resonance imaging; ONFH, osteonecrosis of femoral head.
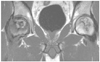
Fig. 4
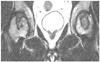
Follow-up MRI showed a decreased lesion size in the right hip. The necrosis of the left femoral head had progressed and was now causing hip pain. MRI, magnetic resonance imaging.
References
1. Meyers MH. Osteonecrosis of the femoral head. Pathogenesis and long-term results of treatment. Clin Orthop Relat Res. 1988. 51–61.
2. Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995. 77:459–474.

3. Hungerford DS, Mont MA. Urbaniak JR, Jones JP, editors. The Role of Core Decompression in the Treatment of Osteonecrosis of the Femoral Head. Osteonecrosis: etiology, diagnosis and treatment. 1997. Rosemont, IL: American Academy of Orthopaedic Surgeons;287–292.
4. Katz RL, Bourne RB, Rorabeck CH, McGee H. Total hip arthroplasty in patients with avascular necrosis of the hip. Follow-up observations on cementless and cemented operations. Clin Orthop Relat Res. 1992. (281):145–151.
5. Mont MA, Marulanda GA, Seyler TM, Plate JF, Delanois RE. Core decompression and nonvascularized bone grafting for the treatment of early stage osteonecrosis of the femoral head. Instr Course Lect. 2007. 56:213–220.
6. Mont MA, Jones LC, Sotereanos DG, Amstutz HC, Hungerford DS. Understanding and treating osteonecrosis of the femoral head. Instr Course Lect. 2000. 49:169–185.
7. Steinberg ME, Bands RE, Parry S, Hoffman E, Chan T, Hartman KM. Does lesion size affect the outcome in avascular necrosis? Clin Orthop Relat Res. 1999. 262–271.

8. Camp JF, Colwell CW Jr. Core decompression of the femoral head for osteonecrosis. J Bone Joint Surg Am. 1986. 68:1313–1319.

9. Koo KH, Kim R, Ko GH, Song HR, Jeong ST, Cho SH. Preventing collapse in early osteonecrosis of the femoral head. A randomised clinical trial of core decompression. J Bone Joint Surg Br. 1995. 77:870–874.

10. Marcus ND, Enneking WF, Massam RA. The silent hip in idiopathic aseptic necrosis. Treatment by bone-grafting. J Bone Joint Surg Am. 1973. 55:1351–1366.
11. Mont MA, Einhorn TA, Sponseller PD, Hungerford DS. The trapdoor procedure using autogenous cortical and cancellous bone grafts for osteonecrosis of the femoral head. J Bone Joint Surg Br. 1998. 80:56–62.

12. Stulberg BN, Davis AW, Bauer TW, Levine M, Easley K. Osteonecrosis of the femoral head. A prospective randomized treatment protocol. Clin Orthop Relat Res. 1991. (268):140–151.
13. Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, et al. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001. (386):71–78.

14. Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004. 86-A:1153–1160.
15. Gangji V, Hauzeur JP, Schoutens A, Hinsenkamp M, Appelboom T, Egrise D. Abnormalities in the replicative capacity of osteoblastic cells in the proximal femur of patients with osteonecrosis of the femoral head. J Rheumatol. 2003. 30:348–351.
16. Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002. 14–23.

17. Hernigou P, Beaujean F. Abnormalities in the bone marrow of the iliac crest in patients who have osteonecrosis secondary to corticosteroid therapy or alcohol abuse. J Bone Joint Surg Am. 1997. 79:1047–1053.

18. Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999. 81:349–355.

19. Yoshioka T, Mishima H, Akaogi H, Sakai S, Li M, Ochiai N. Concentrated autologous bone marrow aspirate transplantation treatment for corticosteroid-induced osteonecrosis of the femoral head in systemic lupus erythematosus. Int Orthop. 2011. 35:823–829.

20. Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995. 77:34–41.

21. Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008. 90:477–484.

22. Ficat RP. Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br. 1985. 67:3–9.

23. Hungerford DS, Zizic TM. . Pathogenesis of ischemic necrosis of the femoral head. In the hip proceeding of the eleventh open scientific
meeting of the Hip Society. 1983. Toronto: CV Mosby;219–262.
24. Launder WJ, Hungerford DS, Jones LH. Hemodynamics of the femoral head. J Bone Joint Surg Am. 1981. 63:442–448.

25. Merle D'Aubigné R, Postel M, Mazabraud A, Massias P, Gueguen J, France P. Idiopathic necrosis of the femoral head in adults. J Bone Joint Surg Br. 1965. 47:612–633.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download