Abstract
Purpose
To evaluate the feasibility for gold immunochromatographic assay (GICA) in rapid detection of influenza virus A infection.
Materials and Methods
Seventy-three patients were enrolled. All patients contributed nasopharyngeal secretions and paired serum samples. Nasopharyngeal secretions was used for colloidal gold immunochromatographic rapid assay for influenza A virus immediately after the collection of specimen. Paired serum samples were used for the hemagglutination inhibition assay at the Centers for Disease Control and Prevention influenza network laboratory in Beijing.
Results
Compare GICA test to hemagglutination inhibition (HI) assay, the Kappa value was 0.402 and the p value in the paired χ2 test was higher than 0.05. Therefore, the difference was not statistically significant. The sensitivity of GICA was 50.0% and the specificity was 90.2%, and the negative predictive value was 90.2%.
Influenza is induced by the influenza virus, which can be divided into type A, B and C according to the characteristics of nucleoprotein and matrix protein of the virus.1 Among them influenza A virus is the most frequently seen virus with the highest virulence.2 Influenza A causes both pandemics and epidemics. Humankind had suffered 3 times influenza pandemics induced by influenza A in 20 century,3 which killed millions of people. A subtype that had never been reported was a disassociation from infectious patients in Mexico and identified as a novel type A H1N1 Influenza virus strain in April 2009, and subsequently this virus spread to more than 90 countries.4-6
Diagnosis and treatment on influenza normally rely on the clinical experiences of doctors, which sometimes can lead to misdiagnosis and antibiotic abusage. Expected clinical symptoms and laboratory examinations are badly needed for the diagnosis of early influenza, which has important significance for controlling the spread of influenza7 and reducing the hazards of diseases and antibiotic abusage.8 Currently, use of the rapid influenza test is recommended by the World Health Organization.9
Cell culture, PCR and hemagglutination inhibition (HI) assay in serological detection have been well received as the "golden criteria" for confirming the infection of influenza virus. However, these methods are time and labor consuming, the requirements on technical conditions are high and thus not suitable for fundamental hospitals.10 Colloidal gold immunochromatographic assay (GICA) with membrane as the solid carrier is a kind of in vitro diagnostic technique that has been developed in recent years, this method has been used in the diagnosis of clinical diseases due to its convenience.11,12 Currently, there are several commercial influenza rapid diagnostic test kits available on the market, such as Binax NOW FluA and Flu B, Directigen Flu A , Flu OIA and QuickVue Influenza Test. However, all of these kits are relatively expensive and difficult to be widely used in resource-limited countries where the threat of influenza epidemic is generally greater and rapid tests are needed the most.13 Recently, a rapid influenza A virus test based on GICA assay was developed by ASCLE BioEngineering Company. The cost of this test is only a quarter of the currently available commercial influenza rapid testing kits. In a previous study, we compare the GICA kit result with the RT-PCR and viral culture, it showed that the consistency between the GICA test and virus culture assay is moderate, in reference to RT-PCR, GICA test demonstrated considerable high sensitivity (74%) and specificity (86%), with Kappa value being 0.61 and overall accuracy of 81%.13 In this study, we compared the result of GICA kit made by ASCLE BioEngineering, Inc. with serum hemagglutination inhibition antibody (HI antibody) because HI tests have been important for the epidemiological surveillance of influenza virus and has been received well by the scientific community.
Seventy three patients showing influenza like symptoms in the Department of Respiratory System in the General Hospital of Beijing military district from September 20, 2009 to March 20, 2010 were enrolled. The entities included acute upper respiratory tract infection, acute bronchitis, pneumonia, bronchial asthma, chronic cor pulmonale and others.
Nasopharyngeal secretions swabs were prepared upon hospitalization or in the early morning on the next day from the patients. On-spot GICA rapid detection was carried out and paired serum samples in the acute stage and the convalescence stage were also collected (1 mL each; interval of 10-18 days). The frozen paired serum samples from the 73 cases were sent to the laboratory in Beijing Disease Prevention and Control Center at 4℃ in different batches, and the HI was carried out to detect three kinds of subtypes of HI antibody of Influenza A (novel H1N1, seasonal H1N1 and seasonal H3N2) within 24 hours. The tests as mentioned above were approved by the ethics committee of the hospital.
GICA-based Influenza virus A Rapid Test Kits (GICA) were developed by ASCLE BioEngineering Company (National Drug Approval #: S20063095, Beijing, China). This test is a double antibody sandwich immunoassay that includes core protein monoclonal antibody and colloidal gold-labeled core antigen monoclonal antibody. In this study, the tests were all performed according to the manufacturer's instructions, as follows: specimens were collected from the nasopharyngeal with sterile nasal swab, which was then fully pressed in a plastic pipe with diluents to solve the secretions. The solution was then dropped onto the test card, and the result was read after 15 minutes. If only the quality control line is a red color, the result is negative; if both the quality control line and the test line were red, the result is positive (Fig. 1).
HI method was used to detect the HI antibodies in three kinds of influenza subtypes, namely novel H1N1, seasonal H1N1 and seasonal H3N2. The antigen virus strains (Influenza virus antigen A1/Tianjin Jinnan/15/2009, A/SiChuan/SW1/2009, A3/Fujian Tongan/196/2009) and the materials for HI methods were all provided by Beijing Disease Prevention and Control Center. Antibodies at no lower than 1 : 40 were considered protective, the quotient in paired serum antibody level the convalescence stage/the acute stage of no lower than four times was considered as a positive result (golden criteria), and the result with the antibody level lower than 1 : 10 were replaced by 1 : 5.14
A database was established and statistical analysis was performed using SPSS for Windows 15.0. The Kappa value was calculated and consistency checking was carried out on the two kinds of methods. Chi-squared tests were carried out on the matched-pairs design, and sensitivity, specificity, positive predict value, negative predict value and Youden index of GICA method was calculated. Chi-squared tests were carried out on the numeration data.
There were 73 patients enrolled in this study. Among them, 41 patients were male, 32 were female, mean age was 61.79 years (SDs 21.19).
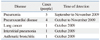
The nasopharyngeal secretions swab samples from 73 patients showing influenza like symptoms were subjected to rapid detection on Influenza A using the GICA method, 12 cases were positive, the positive rate was 16.4% (12/73). The positive patients included the following diagnoses: pneumonia (n=5), pneumocardial disease (n=4), lung cancer (n=1), interstitial pneumonia (n=1), asthmatic bronchitis (n=1). The positive influenza patients were identified mainly during the September, October of 2009 (Table 1).
Twelve of the 73 patients show paired serum antibody level increased ≥4 times with half of them being novel H1N1 positive. Twenty-seven of the 73 patients show paired serum antibody level increased 2 times and most of them had season influenza (Table 2).
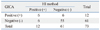
The 73 patients were subjected to rapid detection on Influenza A by using GICA method and paired serum antibody method, and the detection results from the two kinds of methods were subjected to McNemar2 test, p=1.000. The difference was not statistically significant. Consistency was checked using two kinds of methods: the Kappa value=0.402, indicating relatively good consistency. The results are shown in Table 3.
Compared with the positive results in paired serum antibody, the sensitivity for rapid detection by using GICA was 50.0% and the specificity was 90.2%, the positive predict value was 50%, the negative predict value was 90.2% and the Youden index was 0.40.
The comparison of the GICA method and paired serum antibody method showed that the consistency of these two kinds of methods were relatively good. If a patient with a fever was found to be positive for the Influenza A antigen using the GICA method and his specific antibody to paired serum A ratio showed an increase of four times at the same time, it was considered that Influenza A was the pathogen for the morbidity this time, but not the colonized virus in upper respiratory tract.
In this study, the positive results for the Influenza A antigen detection by using GICA method were mostly found in the group of pneumonia and chronic cor pulmonale patients, which was in accordance with the claim in the diagnostic criteria for type A influenza by the Ministry of Public Health in China,15 that people suffering from chronic respiratory disease are high-risk populations. The time of detection was mainly between September 2009 and March 2010, which was also in accordance to the spreading regularity of Influenza A. Furthermore, it was confirmed that the subtypes of novel type A H1N1 influenza in the Influenza A infected cases accounted for more than 50% of the cases, which satisfied the tendency that the novel type A H1N1 virus was the same as the epidemic strain in 2009. However, the cases showed an increase of two times the paired serum antibody and were mostly seasonal type A H1N1 and H3N2, indicating that diverging and spreading of seasonal type A influenza were not negligible.
In conclusion, sensitivity for Influenza A antigen detection based on GICA is relatively low and the specificity is relatively satisfactory, but it is a rapid and convenient method for diagnosis influenza A. This GICA-based influenza A rapid test may be helpful in managing influenza A epidemics in poorly resourced countries and community hospitals where PCR tests cannot be conveniently performed.
Figures and Tables
Fig. 1
Direct-viewing chart for the detection results by using GICA. GICA, gold immunochromatographic assay.

Table 1
Distribution in Entities and Time of Detection in the Positive Results in the Rapid Detection by Using GICA Method
References
1. Lamb RA, Krug RM. Orthomyxoviridae: the viruses and their replication. Fields Virology. 2001. Philadelphia (PA): Lippincott Williams & Wilkins;1487–1531.
2. Hindiyeh M, Levy V, Azar R, Varsano N, Regev L, Shalev Y, et al. Evaluation of a multiplex real-time reverse transcriptase PCR assay for detection and differentiation of influenza viruses A and B during the 2001-2002 influenza season in Israel. J Clin Microbiol. 2005. 43:589–595.

4. Guan Y, Vijaykrishna D, Bahl J, Zhu H, Wang J, Smith GJ. The emergence of pandemic influenza viruses. Protein Cell. 2010. 1:9–13.

5. Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009. 459:1122–1125.

6. Trifonov V, Khiabanian H, Greenbaum B, Rabadan R. The origin of the recent swine influenza A(H1N1) virus infecting humans. Euro Surveill. 2009. 14:pii: 19193.

7. Dwyer DE, Smith DW, Catton MG, Barr IG. Laboratory diagnosis of human seasonal and pandemic influenza virus infection. Med J Aust. 2006. 185:10 Suppl. S48–S53.

8. Bonner AB, Monroe KW, Talley LI, Klasner AE, Kimberlin DW. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: results of a randomized, prospective, controlled trial. Pediatrics. 2003. 112:363–367.

9. World Health Organization. WHO recommendations on the use of rapid testing for influenza diagnosis. Available at: http://www.who.int/influenza/resources/documents/rapid_testing/en/.
10. Petric M, Comanor L, Petti CA. Role of the laboratory in diagnosis of influenza during seasonal epidemics and potential pandemics. J Infect Dis. 2006. 194:Suppl 2. S98–S110.

11. Heeschen C, Goldmann BU, Moeller RH, Hamm CW. Analytical performance and clinical application of a new rapid bedside assay for the detection of serum cardiac troponin I. Clin Chem. 1998. 44:1925–1930.

12. Watanabe T, Ohkubo Y, Matsuoka H, Kimura H, Sakai Y, Ohkaru Y, et al. Development of a simple whole blood panel test for detection of human heart-type fatty acid-binding protein. Clin Biochem. 2001. 34:257–263.

13. Li X, Chen H, Wei J, Lv N, You L. The evaluation of colloidal gold immunochromatographic assay (GICA) for rapid diagnosis of influenza A disease. Clin Chem Lab Med. 2011. 49:1533–1537.

14. Shafir SC, O'Keefe KA, Shoaf KI. Evaluation of the seroprevalence of influenza A(H1N1) 2009 on a university campus: a cross-sectional study. BMC Public Health. 2011. 11:922.

15. Ministry of Health of the People's Republic of China. Program of diagnosis and treatment of influenza A H1N1(2010). Int J Respir. 2011. 31. (In Chinese).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download