Abstract
Purpose
Cinacalcet is effective for treating refractory secondary hyperparathyroidism (SHPT), but little is known about the response rates and clinical factors influencing the response.
Materials and Methods
A prospective, single-arm, multi-center study was performed for 24 weeks. Cinacalcet was administered to patients with intact parathyroid hormone (iPTH) level greater than 300 pg/mL. Cinacalcet was started at a dose of 25 mg daily and titrated until 100 mg to achieve a serum iPTH level <300 pg/mL (primary end point). Early response to cinacalcet was defined as a decrease of iPTH more than 50% within one month.
Results
Fifty-seven patients were examined. Based on the magnitude of iPTH decrease, patients were divided into responder (n=47, 82.5%) and non-responder (n=10, 17.5%) groups. Among the responders, 38 achieved the primary end point, whereas 9 patients showed a reduction in serum iPTH of 30% or more, but did not reach the primary end point. Compared to non-responders, responders were significantly older (p=0.026), female (p=0.041), and diabetics (p<0.001). Additionally, early response was observed more frequently in the responders (30/47, 63.8%), of whom the majority (27/30, 90.0%) achieved the primary end point. Multivariate analysis showed that lower baseline iPTH levels [odds ratio (OR) 0.96, 95% confidence interval (CI) 0.93-0.99], the presence of diabetes (OR 46.45, CI 1.92-1125.6) and early response (OR 21.54, CI 2.94-157.7) were significant clinical factors affecting achievement of iPTH target.
Secondary hyperparathyroidism (SHPT) is a common complication of dialysis as a consequence of decreased renal function, vitamin D deficiency, and impaired mineral metabolism.1,2 According to previous data, disorders of calcium, phosphate, and vitamin D homeostasis, as well as increased intact parathyroid hormone (iPTH) concentrations are associated with multiple comorbidities including renal osteodystrophy, anemia with erythropoietin resistance, vascular calcification, and cardiovascular disease. In fact, disordered calcium phosphorus homeostasis and resultant SHPT cause significant long-term morbidity and mortality in dialysis patients.3-5 Since a better control of SHPT is associated with a more favorable prognosis, optimal management of SHPT may be one of the principal goals in managing hemodialysis patients.
Treatment modalities of SHPT have evolved based upon new insights into the pathogenesis and clinical features of this disorder. Because of the interdependence of calcium, phosphate, vitamin D, and iPTH, pharmacological combinatory treatment is often necessary in patients with advanced renal dysfunction.4,6,7 Despite combined treatment of SHPT with dietary modification, phosphate binders and vitamin D analogs, however, these therapeutic interventions are not always successful,7-9 furthermore, these treatment could aggravate the calcium and phosphorus disturbance.10,11 Therefore, cinacalcet is increasingly prescribed to treat SHPT in patients with renal impairment.6
Cinacalcet, a second generation calcimimetic, binds to and modulates the calcium sensing receptor (CasR) on the parathyroid gland which increases its sensitivity to extracellular calcium, thereby suppressing PTH secretion without exacerbating hyperphosphatemia or hypercalcemia.3,4,12,13 Several randomized controlled studies have demonstrated the efficacy of cinacalcet in lowering iPTH, calcium, and phosphorus levels compared to conventional treatment regimens.2,4,13,14 For instance, Moe, et al.10 demonstrated the long-term efficacy of cinacalcet for control of SHPT, and Lucchi, et al.15 recently showed that earlier use of cinacalcet could provide better iPTH control in dialysis patients. In addition to its effects on iPTH and mineral metabolism, cinacalcet has also been shown to reduce the risk of parathyroidectomy, fractures, and cardiovascular hospitalization.5,16 Moreover, significant reduction of darbepoetin requirements and improvement of health-related quality of life were also observed with cinacalcet treatment in patients with end stage renal disease.17 With these findings, the use of cinacalcet could be an attractive therapeutic option, but little is known about the response rates and clinical factors influencing its therapeutic response.
This study aimed to evaluate the efficacy and safety of cinacalcet in hemodialysis patients with refractory SHPT. And clinical factors influencing therapeutic response to cinacalcet were also investigated.
This study was designed as a prospective, open-label, single-arm, multi-center study in Korea, consisting of a 4-week pretreatment observation period and a 24-week treatment period, from November 2009 to October 2010. A total of 67 patients with SHPT were recruited from seven university hemodialysis units (12, 15, 10, 10, 5, 5, and 10 subjects were included in each hospital). All patients underwent regular 4-hour hemodialysis treatments three times per week with a mean dialysate calcium concentration of 3.0 mEq/L (=6 mg/dL). Cinacalcet was administered to patients whose serum albumin-corrected calcium concentration was greater than 9.0 mg/dL and had refractory hyperparathyroidism, defined as iPTH level was greater than 300 pg/mL for two consecutive measurements, before study initiation, despite treatment with vitamin D and/or phosphate binders of any type. The interval between the two tests was more than 3 months. Intact PTH levels were measured by immunoradiometric assay (Nichols Institute Diagnostics, San Clemente, CA, USA). There was no upper limit of serum iPTH level for enrollment consideration. Cinacalcet was started at 25 mg per day orally. During the initial 2-month dose titration period, serum iPTH, albumin-corrected calcium and phosphorus levels were measured every 2 weeks. The dose of cinacalcet was increased up to 100 mg if serum iPTH was over 300 pg/mL and albumin-corrected calcium level was over 8.5 mg/dL. When the albumin-corrected calcium level decreased to 8.0 mg/dL, cinacalcet was discontinued or the dose was titrated down to avoid hypocalcemia. Thereafter, cinacalcet was maintained for 4 months (maintenance period) to determine its efficacy and safety. Serum calcium, phosphorus, and iPTH levels were measured every 4 weeks during the maintenance period. Serum calcium was corrected with the serum albumin concentration when the serum albumin level was less than 4.0 g/dL [corrected calcium= measured calcium+(4-measured serum albumin)×0.8].18 In this study, to avoid severe hypocalcemia, the types and doses of phosphate binders were flexibly adjusted according to the treating physician's discretion. However, the dose of vitamin D was fixed or allowed only to reduce. The study was approved by the medical ethics committee of each hospital, and all participants provided written informed consent. All clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki.
To assess parathyroid gland size, a computed tomography of the neck was performed upon study initiation. Using a standard protocol, contiguous axial 0.5- to 1-mm images were acquired after the intravenous (IV) administration of contrast material, with a small field of view from the hyoid bone down to the carina. Non-enhanced images were similarly obtained. The detected parathyroid glands were measured in the transverse, anterior-posterior, and longitudinal dimensions; the longest one was used in this study.
The primary end point was the decrease of serum iPTH levels to 300 pg/mL or less during the study period. The secondary end point was a reduction of serum iPTH levels by at least 30% or more from baseline levels. An early response was defined as a decrease in iPTH levels by greater than 50% from the baseline within 1 month of cinacalcet initiation.
All laboratory parameters are expressed as mean±SD when normally distributed, or median with ranges when values were not normally distributed. Differences between groups were analyzed by Fisher's exact test for categorical data, the independent t-test for continuous parameters, and the Mann-Whitney U test for continuous nonparametric data. A repeated measures analysis of variance (ANOVA) linear model was used to compare laboratory parameters after treatment within each group. In addition, Pearson's correlation analysis was used to elucidate the relationship between iPTH levels and sizes of the parathyroid gland. Logistic regression was used to identify factors that predicted a significant reduction in iPTH levels. p values <0.05 were considered statistically significant.
Of the 67 patients initially included in the study, 10 patients were excluded for the following reasons: one patient underwent kidney transplantation before starting cinacalcet, two patients died, and seven patients were dropped out according to the treating physician's discretion (Fig. 1). The remaining 57 patients had a mean age of 50.9±12.5 years, and median duration of dialysis dependence of 11.1 years. At enrollment, 55/57 (96%) patients were receiving phosphate-binding agents, 25/57 (43.8%) were receiving vitamin D sterols, and 32/57 (45.6%) were not being treated with vitamin D agents (because of hypercalcemia, hyperphosphatemia, or both) (Table 1). The type of vitamin D treatment was IV calcitriol and median dose was 2.8 mcg per week. The maximal length detected for the parathyroid gland was highly correlated with the serum iPTH level (r=0.521, p<0.001) (Fig. 2).
The primary end point was achieved in 38 (66.7%) patients. Twenty-eight (73.7%) patients reached the primary end point during the dose titration period, and 10 (26.3%) patients reached the primary end point during the maintenance period. The mean duration of treatment and cinacalcet dose required were 54 days and 37.5 mg, respectively. The median iPTH level decreased from 616.5 to 206.0 pg/mL (55.6% reduction) throughout the study period (Fig. 3). Although the primary end point was not reached in 9 patients (15.8%), these patients showed a reduction in plasma iPTH of at least 30%, thus meeting criteria for the secondary end point. The median iPTH level of the secondary end point group decreased from 822.5 to 383.0 pg/mL, and the mean iPTH level decreased by 43.4% (Fig. 3). Among those patients who reached either the primary or secondary end point (all responders), an early response was observed in 30/47 (63.8%) patients, most of whom 27/30, 90.0% achieved the primary end point. Baseline clinical characteristics of patients with primary end point were similar to those with secondary end point, except that the former were significantly older (54.5±12.1 vs. 44.9±12.6, p=0.041), had lower iPTH levels (616.5 vs. 822.5, p=0.001) and smaller parathyroid gland size (14.2 vs. 19.6 mm, p=0.024). The cumulative proportion of patients to reach primary or secondary end point per month is shown in Fig. 4.
No effect of cinacalcet was seen in 10/57 (17.5%) patients (non-responders); indeed, iPTH levels in these patients were not decreased, or even increased, during cinacalcet treatment. Among non-responders, 5 patients did not response to relatively high-doses of cinacalcet (75-100 mg), however, the other five patients were treated with only 50 mg of cinacalcet because of hypocalcemia during treatment period. Although baseline iPTH levels and the largest length of the parathyroid gland were similar to those of responders, non-responders to cinacalcet were significantly younger (43.0±7.7 vs. 52.6±12.7, p=0.026), male (70.0% vs. 34.0%, p=0.041), and non-diabetic (100.0% vs. 72.3%, p<0.001) (Table 1).
Corrected serum calcium levels also changed significantly during cinacalcet treatment. Interestingly, a reduction in serum calcium concentration was observed in all patients regardless of iPTH response; even non-responders showed significantly decreased serum calcium levels with cinacalcet treatment. Repeated measures ANOVA was conducted to compare the changes of serum calcium levels between the three groups. Though calcium levels were reduced in all three groups (p<0.001), no intergroup differences were identified (Fig. 5, upper). In addition, although serum phosphorus levels also decreased with the use of cinacalcet, the difference was not statistically significant and probably due to concomitant changes in phosphate binder and reductions of vitamin D agents over the study period (Fig. 5, middle). The changes of calcium-phosphorus products were statistically insignificant (Fig. 5, lower).
We investigated clinical factors predicting a better response to cinacalcet treatment using logistic regression analysis. In univariate analysis, older age, diabetes, lower baseline iPTH levels, and an early response to cinacalcet were significantly associated with the achievement of the primary end point. As patients get older, the median iPTH level was decreased, therefore, older patients seem to have better response to cinacalcet (Supplemental Fig. 1). A larger size detected for the parathyroid gland appeared to negatively influence the possibility of reaching the primary end point, however, this observation was statistically insignificant (p=0.101). According to multivariate analysis, lower baseline iPTH levels, diabetes, and the presence of an early response were significant clinical factors affecting achievement of iPTH end points by cinacalcet (Table 2, Supplemental Fig. 2). The response rates according to the range of baseline iPTH level are shown in Fig. 6. The presence of early response was associated with about 20-fold higher probability to achieve target iPTH level. In addition, diabetic patients showed more favorable response to cinacalcet therapy. In fact, nearly all the diabetic patients reached the primary end point (Fig. 7).
Cinacalcet, at doses ranging from 25 to 100 mg once per day, was generally well tolerated, and no patient experienced a serious adverse event during the study period. Adverse events that occurred with ≥5% frequency were nausea/vomiting (21.1%), dyspepsia (14.0%), myalgia/arthralgia (10.5%), constipation (7.0%), febrile sensation (7.0%), and diarrhea (5.3%) (Table 3). Most of these events occurred in a dose-independent manner and were mild to moderate in severity. These symptoms either resolved spontaneously or improved quickly with intervention. In our study, there was no case of albumin-corrected calcium level <7.5 mg/dL. However, 15 patients (26.3%) experienced the decrease of calcium <8.0 mg/dL during study period.
Currently, cinacalcet is the only available potent calcimimetic for the management of SHPT.3 Previous randomized controlled studies have demonstrated its safety and efficacy for controlling serum iPTH, calcium, and phosphorus levels compared to conventional methods.2,4,14 Moreover, recent observational studies suggest the benefit of cinacalcet treatment in reducing all-cause and cardiovascular mortality in dialysis patients.1 However, not all patients have a favorable response to cinacalcet or are able to tolerate the drug. In this study, therefore, we prospectively investigated 67 hemodialysis patients from seven medical centers in Korea and evaluated the response rate to cinacalcet. Depending on the degree of response, patients were divided into two groups: responders (further subdivided to patients with primary or secondary end points), and non-responders.
One major finding of this study was that more than 80% of patients showed a good response to cinacalcet, with the majority achieving the iPTH target levels recommended by the Kidney Disease Outcomes Quality Initiative.19 Although only 20% of responders reached secondary end point, their baseline iPTH levels were significantly higher than those who met the primary end point, and furthermore, six patients could not receive full-doses of cinacalcet because of hypocalcemia. Therefore, patients with hypocalcemia are expected to have insufficient doses of cinacalcet. Treatment over longer durations and with higher doses may lead to the primary end point in these patients as well.
On the contrary, cinacalcet had no impact on iPTH reduction in about 17% of patients. Despite half of these patients could not receive a sufficient dose because of hypocalcemia, the remaining half were resistant to full-doses of cinacalcet therapy. Except the fact that non-responders tended to be younger and less frequently diabetic, their baseline characteristics were similar to responders. Even baseline iPTH level and the largest diameter of the parathyroid gland were also comparable between the two groups. Therefore, the prediction of future response rate with baseline characteristics seems to be difficult. However, this finding is not so surprising because the efficacy of cinacalcet for lowering iPTH levels has been reported to be unaffected by disease severity, as judged by baseline iPTH values or adenoma formation. For instance, Frazão, et al.8 reported that cinacalcet could reduce plasma iPTH levels irrespective of the severity of SHPT, and Shoback, et al.20 demonstrated the efficacy of cinacalcet in patients with primary hyperparathyroidism with adenoma. In addition, excellent long-term efficacy in reducing serum iPTH levels was observed also in patients with severe, refractory tertiary hyperparathyroidism with nodular hyperplasia.21,22 Therefore, more large-scale study is required to ascertain detailed characteristics of these responder/non-responder groups. In our study, nevertheless, lower baseline iPTH level was significantly associated with more favorable outcome to cinacalcet treatment. Similarly, a larger size parathyroid gland appeared to negatively influence the possibility of reaching the primary end point, although statistically insignificant. In line with our present finding, Yamamoto, et al.23 recently found that two or more enlarged parathyroid glands might be a significant risk of poor response to cinacalcet treatment.
In this respect, it is of particular interest to note that an early decrease in iPTH may be an important clue for predicting better response to cinacalcet. More than 60% of the responders in our study showed remarkable decrease of iPTH levels by 50% or more within 1 month after the administration of cinacalcet, and most patients with an early response had excellent outcomes. Moreover, multivariate logistic regression analysis revealed that the efficacy of cinacalcet in iPTH target achievement was significantly associated with the presence of an early response, which was consistent regardless of the presence of diabetes. In accordance with our data, a reduction in iPTH levels after 3 months of treatment with relatively lower doses has been reported as a prognostic marker of good response to cinacalcet treatment.24 Another important finding in our study was that diabetic patients showed a more favorable response to cinacalcet compared to non-diabetics (p<0.001). The presence of diabetes was associated with 46-fold increased probability to achieve primary end point, and all the non-responders were non-diabetics, suggesting the possibility that these results are due to unique characteristics of renal osteodystrophy in diabetic patients. Among diabetic patients, it is well known that high-turnover bone disorders are distinctly uncommon, while low-turnover bone disorders are more frequent.25,26 Bone morphology in diabetic patients is characterized by lower trabecular and osteoid bone volume, as well as markedly reduced indices of bone formation and resorption. The small amount of bone and lack of osteomalacia are unique features of diabetic patients with chronic renal disease.27
With cinacalcet treatment, serum calcium levels also decreased significantly and this change has been well-demonstrated in many previous studies.2,14,22 On lowering iPTH, bone turnover is expected to decrease substantially, resulting in less calcium release from bone. Interestingly, however, the significant reduction of serum calcium level was also observed in patients with non-responder group, as well. The reduction of serum calcium levels in these non-responders might be independent of the effect of cinacalcet on the parathyroid gland; rather, it could be attributable to the direct inhibition of calcium-dependent bone metabolism through CaSR expressed in bone cells. Indeed, recent studies have demonstrated that CaSR is expressed in several tissues other than the parathyroid gland,3 and it is well known that CaSR is expressed and regulated in bone, intestinal, renal, and vascular cells.28 The reduction of bone metabolism and histomorphometric resorption parameters during cinacalcet treatment could be explained by both a PTH-dependent decrease of bone turnover, as well as calcium-mediated direct control of calcium fluxes from bones.29
The limitations of this prospective, single-arm study include its open-label design, the relatively small number of patients enrolled, and the lack of both a placebo and a comparison group. Second, half of the patients in the non-responder group could not receive the full dose of cinacalcet because of hypocalcemia. Moreover, the dose of vitamin D agents was either fixed or only allowed to be reduced in our study design; therefore, the potential to reach the primary or secondary end point for iPTH levels with combined high-dose cinacalcet and a vitamin D agent cannot be ruled out. On the contrary, half of non-responders had either no change or an increase in iPTH levels even when using high-dose cinacalcet treatment. Therefore, these patients could be considered as real non-responders or resistant group. Last, we did neither measure parathyroid gland volume nor count the number of enlarged parathyroid glands as possible prognostic indicators for cinacalcet efficacy.
In conclusion, cinacalcet was effective and well tolerated in 82.5% of patients undergoing hemodialysis, whereas 17.5% of patients were resistant to cinacalcet. An early response to cinacalcet (a more than 50% decrease of iPTH within 1 month) was closely associated with a much more favorable response to cinacalcet treatment. In addition, baseline iPTH levels and the presence of diabetes also seem to be important factors for predicting the effect of cinacalcet.
Figures and Tables
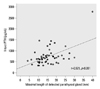 | Fig. 2Parathyroid gland size and serum iPTH levels. Maximal size of parathyroid gland detected on neck CT was highly correlated with serum iPTH levels (r=0.521, p<0.001). iPTH, intact parathyroid hormone. |
 | Fig. 3Effects of cinacalcet treatment on serum iPTH levels. In patients who achieved primary or secondary end point (responders), iPTH levels decreased significantly from baseline, whereas the levels were not changed or mildly increased in the non-responder group. iPTH, intact parathyroid hormone. |
 | Fig. 5Changes in serum calcium, phosphorus and calcium phosphorus products levels with cinacalcet treatment. Serum calcium levels were reduced in all three groups (p<0.001), however, the changes of serum phosphorus and calcium phosphorus products levels were statistically insignificant. Data were analyzed by repeated measures ANOVA. ANOVA, analysis of variance. |
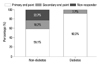 | Fig. 7Response rate to cinacalcet according to diabetes. Diabetic patients showed a more favorable response to cinacalcet compared to non-diabetics (p<0.001). |
 | Supplemental Fig. 1Median iPTH levels and response rate to cinacalcet according to age group. iPTH, intact parathyroid hormone. |
 | Supplemental Fig. 2Clinical factors affecting achievement of iPTH target. iPTH, intact parathyroid hormone; DM, diabetes mellitus. |
Table 1
Baseline Characteristics of All Patients (n=57)

MBP, mean blood pressure; PTG, parathyroid gland; iPTH, intact parathyroid hormone; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CRP, C-reactive protein; AV, arteriovenous.
*Median with ranges.
†p<0.05 between responder vs. non-responder.
‡p<0.05 between primary vs. secondary responder.
References
1. Block GA, Zaun D, Smits G, Persky M, Brillhart S, Nieman K, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int. 2010. 78:578–589.

2. Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004. 350:1516–1525.

3. Cozzolino M, Mazzaferro S, Messa P. New insights into the role of calcium-sensing receptor activation. J Nephrol. 2011. 24:Suppl 18. S38–S41.

4. Moe SM, Chertow GM, Coburn JW, Quarles LD, Goodman WG, Block GA, et al. Achieving NKF-K/DOQI bone metabolism and disease treatment goals with cinacalcet HCl. Kidney Int. 2005. 67:760–771.

5. Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005. 68:1793–1800.

6. Drüeke TB, Ritz E. Treatment of secondary hyperparathyroidism in CKD patients with cinacalcet and/or vitamin D derivatives. Clin J Am Soc Nephrol. 2009. 4:234–241.

7. Kalantar-Zadeh K, Shah A, Duong U, Hechter RC, Dukkipati R, Kovesdy CP. Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney Int Suppl. 2010. S10–S21.

8. Frazão JM, Messa P, Mellotte GJ, Geiger H, Hagen EC, Quarles LD, et al. Cinacalcet reduces plasma intact parathyroid hormone, serum phosphate and calcium levels in patients with secondary hyperparathyroidism irrespective of its severity. Clin Nephrol. 2011. 76:233–243.

9. Young EW, Akiba T, Albert JM, McCarthy JT, Kerr PG, Mendelssohn DC, et al. Magnitude and impact of abnormal mineral metabolism in hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004. 44:5 Suppl 2. 34–38.

10. Moe SM, Cunningham J, Bommer J, Adler S, Rosansky SJ, Urena-Torres P, et al. Long-term treatment of secondary hyperparathyroidism with the calcimimetic cinacalcet HCl. Nephrol Dial Transplant. 2005. 20:2186–2193.

11. Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008. 52:519–530.

12. Goodman WG, Frazao JM, Goodkin DA, Turner SA, Liu W, Coburn JW. A calcimimetic agent lowers plasma parathyroid hormone levels in patients with secondary hyperparathyroidism. Kidney Int. 2000. 58:436–445.

13. Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005. 16:800–807.

14. Akizawa T, Kido R, Fukagawa M, Onishi Y, Yamaguchi T, Hasegawa T, et al. Decreases in PTH in Japanese hemodialysis patients with secondary hyperparathyroidism: associations with changing practice patterns. Clin J Am Soc Nephrol. 2011. 6:2280–2288.

15. Lucchi L, Carboni C, Stipo L, Malaguti V, Ferrari F, Graziani R, et al. Early initiation of cinacalcet for the treatment of secondary hyperparathyroidism in hemodialysis patients: a three-year clinical experience. Artif Organs. 2011. 35:1186–1193.

16. Suzuki H, Inoue T, Watanabe Y, Kikuta T, Sato T, Tsuda M, et al. Does cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism, improve arterial stiffness in patients on continuous ambulatory peritoneal dialysis? Adv Perit Dial. 2011. 27:134–139.
17. Battistella M, Richardson RM, Bargman JM, Chan CT. Improved parathyroid hormone control by cinacalcet is associated with reduction in darbepoetin requirement in patients with end-stage renal disease. Clin Nephrol. 2011. 76:99–103.

18. Wald R, Tentori F, Tighiouart H, Zager PG, Miskulin DC. Impact of the Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guidelines for Bone Metabolism and Disease in a large dialysis network. Am J Kidney Dis. 2007. 49:257–266.

19. National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003. 42:4 Suppl 3. S1–S201.
20. Shoback DM, Bilezikian JP, Turner SA, McCary LC, Guo MD, Peacock M. The calcimimetic cinacalcet normalizes serum calcium in subjects with primary hyperparathyroidism. J Clin Endocrinol Metab. 2003. 88:5644–5649.

21. Kruse AE, Eisenberger U, Frey FJ, Mohaupt MG. Effect of cinacalcet cessation in renal transplant recipients with persistent hyperparathyroidism. Nephrol Dial Transplant. 2007. 22:2362–2365.

22. Komaba H, Nakanishi S, Fujimori A, Tanaka M, Shin J, Shibuya K, et al. Cinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2010. 5:2305–2314.

23. Yamamoto M, Ogata H, Mizobuchi M, Yoshida N, Kumata-Maeta C, Koiwa F, et al. Number of enlarged parathyroid glands might be a predictor of cinacalcet response in advanced secondary hyperparathyroidism. Clin Exp Nephrol. 2012. 16:292–299.

24. Segura Torres P, Borrego Utiel FJ, Sánchez Perales MC, García Cortés MJ, Biechy Baldán MM, Pérez Bañasco V. [Analysis of the efficacy and factors influencing the response of secondary hyperparathyroidism patients on hemodialysis to cinacalcet]. Nefrologia. 2010. 30:443–451.
25. Pei Y, Hercz G, Greenwood C, Segre G, Manuel A, Saiphoo C, et al. Renal osteodystrophy in diabetic patients. Kidney Int. 1993. 44:159–164.

26. Vincenti F, Hattner R, Amend WJ Jr, Feduska NJ, Duca RM, Salvatierra O Jr. Decreased secondary hyperparathyroidism in diabetic patients receiving hemodialysis. JAMA. 1981. 245:930–933.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download