Abstract
Purpose
Endobronchial metastasis is defined as documented extrathoracic malignancies metastatic to the endobronchus within a bronchoscopically visible range. Although the clinical and radiologic findings of endobronchial metastasis are similar to primary lung cancer, treatment and prognosis may be different. We hereby investigated the clinical, radiologic and bronchoscopic aspects of endobronchial metastases (EBM) in Korean patients.
Materials and Methods
A total of 43 patients with EBM who underwent bronchoscopic biopsies from June 1991 to December 2009 at Severance Hospital, Yonsei University College of Medicine in Seoul, Korea, were analyzed retrospectively. We evaluated clinical, radiologic and bronchoscopic characteristics of EBM.
Results
The patients consisted of 27 males and 16 females and their ages ranged from 18 to 77 years. The common primary cancers related to EBM were rectal (16.3%), colon (11.6%), breast (9.3%) and uterine (9.3%) cancers. The mean interval from diagnosis of primary cancer to EBM was 36 months, and the mean survival duration from diagnosis of EBM was 16.1 months in 33 deceased patients.
Conclusion
EBM develop in various types of malignancies at various times with unremarkable manifestations. Therefore, physicians should consider the possibility of EBM, especially if a patient has a history of any malignancy, regardless of respiratory symptoms. Respiratory symptoms related with EBM can be treated by various safe procedures.
Endobronchial lesions have many different histological causes, the most frequent being bronchogenic small cell carcinoma, well-differentiated neuroendocrine carcinoma, bronchogenic squamous cell carcinoma and non-small cell carcinoma.1 Only 1.1% of endobronchial tumors are metastatic.2 However, the incidence of endobronchial metastases (EBM) differs according to the status of disease, patient group, and study program used.3-5 According to the study by Oshikawa, et al.,4 the incidence of EBM was reported as 15 cases (23%) out of 65 patients with metastatic pulmonary disease. In another study by Braman and Whitcomb,3 EBM were readily visible bronchoscopically in the main bronchus and lobar bronchus, and the prevalence of EBM was 2%.
From a histopathologic standpoint, EBM are observed in various types of malignancies including colorectal, breast, kidney, stomach, ovarian, thyroid, uterine, testicular, nasopharynx, prostate, adrenal carcinomas, sarcomas, histocytoma and plasmacytomas.6-10 In addition, many other benign etiologic tumors have been reported, such as fungal disease, inflammatory pseudopolyp, lipoma and broncholith.11 The most common EBM are colorectal, breast and kidney carcinomas.12,13 Although there are many underlying causes, most have similar clinical presentations such as cough, dyspnea and sputum.
Metastases from non-pulmonary malignancies to the lungs are very common, but EBM from extrathoracic malignancy are rare. Moreover, it is difficult to distinguish bronchogenic carcinoma from metastasis of extrathoracic malignancies. However, the treatment modality of EBM is determined by the histologic features of the primary tumor, biologic behavior, anatomic location, evidence of other metastatic sites, present symptoms, patient performance status and life expectancy. Therefore, the accurate diagnosis of EMB is very important for decisions regarding treatment modality.14 Only a small number of cases have been reported in Korea.15-17 The objective of this study was to investigate the clinical, radiologic and bronchoscopic aspects of EMB in Korean patients.
We retrospectively reviewed all fibrobronchoscopic reports from June 1991 to December 2009 at Severance Hospital, Yonsei University College of Medicine, in Seoul, Korea and identified 43 patients who were diagnosed with EMB. We retrospectively reviewed the medical records of all 43 patients and collected data on baseline characteristics, histopathological results, the interval of time from the primary tumor diagnosis to EBM diagnosis, clinical symptoms, radiologic findings, including chest X-ray and computed tomography, developmental modes, treatment modalities, and survival time.
A flexible bronchoscopy was performed by a pulmonologist under sedation for each patient. Histopathologic diagnosis was confirmed in all cases by direct biopsy with bronchoscopy. EBM were defined as bronchoscopically visible involvement of the subsegmental or more proximal central bronchus with histologically verified extrathoracic primary malignancy. Primary lung cancer, esophageal cancer and lymphoma were excluded in this study because these cancers can invade the bronchus directly without metastasis.
The developmental mode of EBM was classified into four groups, according to Kiryu, et al.,18 using chest images, bronchoscopic findings, and histology, as follows: type I, direct metastasis to the bronchus; type II, endobronchial invasion of parenchymal mass; type III, endobronchial invasion of mediastinal or hilar lymphadenopathy; and type IV, extension of peripheral tumor along the proximal bronchus.
This study protocol was approved by the Institutional Review Board of Severance Hospital.
Patient characteristics and presenting symptoms are summarized in Table 1. The 43 patients consisted of 27 males and 16 females. Their ages ranged from 18 to 77, with a mean age of 55.1 years. The most common presenting symptoms were cough in 22 (51.2%) patients and dyspnea in 12 (27.9%) patients. Twelve patients (27.9%) with EBM were asymptomatic. Nine patients were detected by routine follow-up examination, two patients while evaluating other symptoms and one patient by medical check-up.
Clinical findings of patients with endobronchial metastases are shown in Table 2. Common primary cancers related with EMB were rectal (7 patients, 16.3%), colon (5 patients, 11.6%), breast (4 patients, 9.3%) and uterine (4 patients, 9.3%) cancers. The others included stomach, thyroid, hepatoma, melanoma, nasopharynx, osteosarcoma, prostate, kidney, urothelial, fibrosarcoma originating from left calf, salivary gland, parotid gland, cervix, and malignant fibrous histocytoma originating from the left thigh. The primary tumor site could not be detected in one of the patients with melanoma. Thirty-six patients (83.7%) had a previous history of extrathoracic malignancy. In seven patients, EBM were diagnosed at the same time as diagnosis of the following primary tumors: melanoma (2), prostate (2), parotid gland (1), salivary gland (1) and stomach (1) cancers. The interval time from diagnosis of the primary tumor to the diagnosis of EBM was between 0 to 160 months, with a median period of 36 months. The time interval between detection of the primary tumors and EMB was relatively long for thyroid (156 months), breast (111 months) and rectal (96 months) cancers. The treatment modalities were surgery in nine, chemotherapy in 17, radiotherapy in 10, chemotherapy combined with radiotherapy in one, and supportive care in 11 patients. Two patients received chemotherapy and surgery. The mean survival time from diagnosis of endobronchial metastasis was 16.1 months in 33 deceased patients.
There were 50 bronchoscopically visible endobronchial lesions, and their locations are listed in Table 3. EMB were observed at the left bronchus in 21 patients (48.8%) and at the right bronchus in 28 patients (65.1%). One colon cancer patient had three bronchoscopically visible endobronchial lesions. One melanoma, two prostate cancer, one rectal cancer, and one stomach cancer patient had two visible endobronchial lesions.
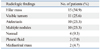
The radiologic findings of 50 EMB are summarized in Table 4. Six patients had multiple lesions. Hilar mass (15 patients, 34.9%) was the most frequent finding on chest radiography, followed by visible tumors (11 patients, 25.6%), atelectasis (10 patients, 23.3%) and multiple nodules (10 patients, 23.3%). Four patients (9.3%) showed normal radiologic findings and three of them had rectal cancer.
Mean survival time after EBM diagnosis in long-term survivors who were treated was 17.4 months, whereas that of patients given supportive care was 12.4 months. Treatment and outcome after EBM diagnosis in some long-term surviors are shown in Table 5. EBM related with thyroid, breast or rectal cancer appear less aggressive than in other malignancies. One of the thyroid cancer patients who received a total thyroidectomy due to recurrence six years after initial partial thyroidectomy showed re-recurrence as EBM after an additional seven-year period. One patient with hepatocellular carcinoma received chemotherapy, radiotherapy and surgery, and he lived for more than ten years after these multidisciplinary treatments. One patient with rectal cancer received photodynamic therapy and one patient with thyroid cancer received radioactive iodine therapy.
Classifications of the developmental modes of endobronchial metastases and the involvement of other organs are shown in Table 6. Patients were divided into four groups according to the classification of developmental modes: type I, 16 patients (37.2%); type II, 12 patients (27.9%); type III, 6 patients (14.0%); and type IV, 9 patients (20.9%). In 13 patients, extrathoracic metastases were present at the same time as EBM diagnosis. The most common extrathoracic metastatic site was bone.
In this study, we analyzed the clinical, radiologic and bronchoscopic aspects of EBM in Korean patients. EBM were most frequently detected in colorectal, breast and uterine cancers and the main bronchus was the most common site of the primary tumor. Cough and a hilar mass on radiography were the most frequent clinical manifestations.
EBM from an extrathoracic primary tumor are very rare.13 When an endobronchial mass is detected, it is important to distinguish primary lung cancer from lung metastasis of extrathoracic primary tumors. Primary lung cancer and EBM have different prognoses, so diagnosis is very important to select treatment modalities for individual patients. However, EBM are probably underestimated, because bronchoscopy is not used routinely in patients with malignancy history. Staining bronchoscopy, macroscopic bronchoscopy or auto-fluorescence bronchoscopy can be used to detect less invasive endobronchial tumors early, thus allowing earlier treatment and a better prognosis. Moreover, electronic noses are also available in early detection of bronchogenic carcinoma.19 In many studies, EBM are clinically, radiologically and bronchoscopically indistinguishable from bronchogenic carcinoma in most cases. They should be confirmed by histological analysis and the pathologic comparison of EBM and the primary site is important. Rosenblatt, et al.20 showed that the distinct features of metastatic bronchial lesions in the early stage included an intact epithelium covering the tumor mass with subepithelial lymphatics. Clear cell renal cancer is easily diagnosed by immunohistochemistry using markers such as CK7, CK20, thyroid transcription factor-1 and apoprotein-A1.21-23 These molecular biologic tools are very useful to distinguish extrathoracic lung metastasis from primary lung cancer.
The interval between diagnosis of primary cancer and detection of EBM is reported to be about 50 months and our study revealed an average interval of 36 months.13 This period was long compared to the average intervals for metastasis to other organs in these cancers.24 This might mean that EBM frequently occur in "well-controlled" primary cancer. However, there is no obvious explanation for this phenomenon. As our study demonstrated, EBM may not indicate a poor prognosis and should not be thought a bad prognostic factor in choosing treatment modalities. Akoglu, et al. showed that mean survival time was longer in patients with chemotherapy or radiotherapy than in patients with supportive care.14 After diagnosis of EBM, the median survival duration was 16.1 months in our study, similar to the 15.5 months found by Kiryu, et al.18 Larger studies may be required to evaluate molecular markers for genes that would explain the characteristics of these time intervals.
EBM are known to manifest late in the course of cancer progression. However, there are cases of lesions being diagnosed at the same time as primary tumors.25 In our study, EBM and primary cancer were diagnosed simultaneously in seven patients with melanoma, prostate, parotid, salivary and stomach cancers. The most common metastatic sites of melanoma are lung and liver, and EBM were found at the same time as diagnosis of all melanomas in this study.26 Moreover, prostate cancer is commonly diagnosed simultaneously with other organ metastasis including EBM because it is relatively indolent and slow-growing compared to other malignancies. Breast cancer and colorectal cancer are the most frequently reported endobronchial metastatic cancers, probably because they have high incidences and good prognoses, compared to other malignancies. Head and neck cancers such as parotid and salivary gland cancers were also found to frequently cause EBM in this study. Therefore, although physicians should suspect the possibility of EBM in all oncologic patients, more attention should be paid to patients with breast cancer, colorectal cancer and kidney cancer. It remains unclear why EBM are more frequently detected in these tumors.
Several studies reported that asymptomatic patients range from 20 to 62.5%.13,18,27 In our study, there were 12 patients (27.9%) who were asymptomatic. This result suggests that physicians should consider the possibility of EBM when they encounter patients previously diagnosed with cancer and with manifested respiratory symptoms. Moreover, regular follow-up is important, even in patients without any respiratory symptoms.
Atelectasis and visible tumors with hilar masses are common manifestations observed in the chest radiographs of patients with EBM.13,28 In our study, hilar mass, visible tumor and atelectasis were common findings, but 9.3% of patients had normal chest X-rays. Therefore, physicians need to pay attention to the overall symptoms of patients with a history of malignancy and keep the possibility of endobronchial metastasis in mind.
Most of our treatments of EBM were limited to conventional anti-cancer therapy such as chemotherapy, radiotherapy, and surgery; however, there are various treatment methods to relieve dyspnea, hemoptysis or obstructive pneumonia caused by endobronchial metastasis. Intrabronchial therapy such as stent insertion, brachytherapy, and photodynamic therapy, or laser evaporation have been performed.13 These procedures are safe and effective as palliative treatment and can prolong survival in selected patients.29 One patient with rectal cancer and EBM was treated with phototherapy and showed good prognosis in the aspect of survival duration and symptom control.
Kiryu, et al.18 divided EBM patients into mode types of metastasis according to four developmental conditions and type IV was the most common condition. However, in our study, type I was most common, probably because it is difficult to differentiate type II and IV.14 Our experience suggests that a more reliable classification of the mode of metastatic spread is needed.
The present study had several limitations. First, the types of primary tumors with EBM varied in this study, so overall prognosis would not represent the characteristics of each malignancy. Second, there were many improvements in the diagnosis and treatment of malignancies during this study period between 1991 and 2009. These improvements would influence the disease progression even in the same type of malignancy.
In conclusion, this study suggests that EBM develop in various types of malignancies at various times. However, the incidence of EBM has been underestimated because of its unremarkable manifestations. Some patients with EBM show no clinical symptoms or normal image findings. However, EBM should be distinguished from primary lung cancer through histological confirmation, and bronchoscopy in these asymptomatic or radiologically free patients with malignancy is useful. Moreover, respiratory symptoms of EBM may be treated by various safe procedures, and in some cases, intrabronchial therapy may prolong patient survival.7 Therefore, physicians should consider the possibility of EBM when they encounter patients with colorectal or breast cancer, especially when respiratory symptoms are present.
Figures and Tables
Table 2
Clinical Findings in 43 Patients with Endobronchial Metastases Diagnosed by Bronchoscopy with Biopsy
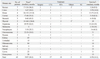
Table 4
Radiologic Findings in 43 Patients with Endobronchial Metastasis Diagnosed by Bronchoscopy with Biopsy
References
1. Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: analysis of 32 cases. J Surg Oncol. 1996. 62:249–252.

2. Kreisman H, Wolkove N, Finkelstein HS, Cohen C, Margolese R, Frank H. Breast cancer and thoracic metastases: review of 119 patients. Thorax. 1983. 38:175–179.

4. Oshikawa K, Ohno S, Ishii Y, Kitamura S. Evaluation of bronchoscopic findings in patients with metastatic pulmonary tumor. Intern Med. 1998. 37:349–353.

6. Amer E, Guy J, Vaze B. Endobronchial metastasis from renal adenocarcinoma simulating a foreign body. Thorax. 1981. 36:183–184.

7. Fournel C, Bertoletti L, Nguyen B, Vergnon JM. Endobronchial metastases from colorectal cancers: natural history and role of interventional bronchoscopy. Respiration. 2009. 77:63–69.

9. Hanyu T, Kanda T, Matsuki A, Hasegawa G, Yajima K, Tsuchida M, et al. Endobronchial metastasis from adenocarcinoma of gastric cardia 7 years after potentially curable resection. World J Gastrointest Surg. 2010. 2:270–274.

10. Shen Q, Yao Y, Teng X, Zhou J. Endobronchial metastasis from prostate cancer mimicking primary lung cancer. Intern Med. 2010. 49:1613–1615.

11. Magro CM, Ross P Jr. Endobronchial mimics of primary endobronchial carcinoma: a clinical study of 25 cases. Can Respir J. 2005. 12:123–127.

12. Berg HK, Petrelli NJ, Herrera L, Lopez C, Mittelman A. Endobronchial metastasis from colorectal carcinoma. Dis Colon Rectum. 1984. 27:745–748.

13. Sørensen JB. Endobronchial metastases from extrapulmonary solid tumors. Acta Oncol. 2004. 43:73–79.

14. Akoglu S, Uçan ES, Celik G, Sener G, Sevinç C, Kilinç O, et al. Endobronchial metastases from extrathoracic malignancies. Clin Exp Metastasis. 2005. 22:587–591.

15. Kim YS, Chang J, Kim YS, Shin DH, Kim HS, Kim SK, et al. Endobronchial metastasis of uterine cervix cancer: a two case reports and a review of the literature. Yonsei Med J. 2002. 43:547–552.

16. Lee KY, Ryu SJ, Joo M. Endobronchial metastasis of hepatocellular carcinoma. Yonsei Med J. 2003. 44:544–547.

17. Park YB, Byun YS, Kim SK, Yang DG, Chang J, Kim JH, et al. Endobronchial metastasis from stomach cancer. Respirology. 1999. 4:89–92.

18. Kiryu T, Hoshi H, Matsui E, Iwata H, Kokubo M, Shimokawa K, et al. Endotracheal/endobronchial metastases: clinicopathologic study with special reference to developmental modes. Chest. 2001. 119:768–775.
19. Machado RF, Laskowski D, Deffenderfer O, Burch T, Zheng S, Mazzone PJ, et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am J Respir Crit Care Med. 2005. 171:1286–1291.

20. Rosenblatt MB, Lisa JR, Collier F. Criteria for the histologic diagnosis of bronchogenic carcinoma. Dis Chest. 1967. 51:587–595.

21. Kummar S, Fogarasi M, Canova A, Mota A, Ciesielski T. Cytokeratin 7 and 20 staining for the diagnosis of lung and colorectal adenocarcinoma. Br J Cancer. 2002. 86:1884–1887.

22. Srodon M, Westra WH. Immunohistochemical staining for thyroid transcription factor-1: a helpful aid in discerning primary site of tumor origin in patients with brain metastases. Hum Pathol. 2002. 33:642–645.

23. Wilson RW, Moran CA. Primary melanoma of the lung: a clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol. 1997. 21:1196–1202.

24. Coriat R, Diaz O, de la Fouchardière C, Desseigne F, Négrier S. Endobronchial metastases from colorectal adenocarcinomas: clinical and endoscopic characteristics and patient prognosis. Oncology. 2007. 73:395–400.

25. Dursun AB, Demirag F, Bayiz H, Sertkaya D. Endobronchial metastases: a clinicopathological analysis. Respirology. 2005. 10:510–514.

26. Chen JT, Dahmash NS, Ravin CE, Heaston DK, Putman CE, Seigler HF, et al. Metastatic melanoma in the thorax: report of 130 patients. AJR Am J Roentgenol. 1981. 137:293–298.

27. Heitmiller RF, Marasco WJ, Hruban RH, Marsh BR. Endobronchial metastasis. J Thorac Cardiovasc Surg. 1993. 106:537–542.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download