Abstract
Purpose
After esophagectomy and gastric reconstruction for esophageal cancer, patients suffer from various symptoms that can detract from quality of life. Endoscopy is a useful diagnostic tool for evaluating patients after esophagectomy. This observational study was performed to investigate the correlation between symptoms and endoscopic findings one year after esophageal surgery and to assess the clinical usefulness of one-year endoscopic follow-up.
Materials and Methods
From 2001 to 2008, 162 patients who underwent esophagectomy with gastric reconstruction were endoscopically examined one year after operation.
Results
Patients suffered from the following symptoms: nocturnal cough (n=10), regurgitation (n=7), cervical heartburn (n=3), lump sensation (n=2), dysphagia (n=20) and odynophagia (n=22). Eighty-five (52.5%) patients had abnormal findings on endoscopic examination. Twelve (7.4%) patients had reflux esophagitis, and 37 (22.8%) patients had an anastomotic stricture. Only stricture-related symptoms were correlated with the finding of anastomotic strictures (p<0.001). Two patients had recurrences at the anastomotic sites, and four patients had regional lymph node recurrences with gastric conduit invasion visualized by endoscopy. Newly-developed malignancies in the esophageal remnant or hypopharynx that were not detected by clinical symptoms and imaging studies were reported in two patients.
Recent advances in the treatment of esophageal cancer have led to better prognoses, and increased attention has been focused on quality of life after an esophagectomy as a result. Most patients who undergo an esophagectomy suffer from various symptoms that may diminish their quality of life. These symptoms include dysphagia, heartburn, regurgitation, early satiety, fatigue and psychological problems. According to a previous report, only 16% of patients are asymptomatic after an esophagectomy, 60% of patients suffer from reflux symptoms, and 25% patients suffer from dysphagia symptoms.1 Several diagnostic tools have been used to evaluate patients after esophagectomy, and endoscopy is one of the most useful of these tools. However, clinical symptoms and endoscopic findings are not closely correlated in patients who undergo an esophagectomy.2 Some patients suffer from dysphagia, odynophagia or reflux symptoms without any endoscopic evidence of stricture or reflux, whereas other patients have no clinical symptoms even when there are endoscopic findings of strictures or reflux. Most centers perform endoscopic evaluations for symptomatic patients only; therefore, the true incidence of abnormal findings on endoscopy after esophagectomy might be underestimated.3
Few studies have reported on follow-up examinations of the remnant esophagus and esophageal anastomosis following esophagectomy with gastric reconstruction.4 Regular endoscopic follow-up is not a routine procedure at some clinical centers. However, we have performed routine one-year endoscopic follow-up after esophagectomy and gastric reconstruction since 2001. Therefore, we examined the results of routine one-year follow-up endoscopic evaluations in patients who underwent gastric reconstruction after an esophagectomy. Then, we investigated the relationship between clinical symptoms and endoscopic findings; moreover, we also assessed the usefulness of endoscopic follow-up after esophagectomy and gastric reconstruction.
This protocol was reviewed by the institutional review board and approved as a retrospective study (NCCNCS-10-408) that did not require individual consent according to institutional guidelines. From 2001 to 2008, 162 patients underwent endoscopy one year after their operation. The prospectively collected medical records were retrospectively reviewed along with the esophagogastroduodenoscopy (EGD) findings.
Neoadjuvant therapy was not performed in this series. For patients with middle and lower esophageal cancers, a two-field lymph node dissection and intrathoracic esophagogastrostomy were performed using the whole stomach as a conduit, and an anastomosis was performed with a 28-mm end-to-end anastomosis stapler (EEA stapler; Autosuture, U.S. Surgical Corp., Norwalk, CT, USA). For patients with upper esophageal cancer, a three-field lymph node dissection was routinely performed. If an intrathoracic esophagogastrostomy was possible, an intrathoracic anastomosis was performed using the entire stomach. For all others, a cervical esophagogastrostomy with a gastric tube was performed. The gastric tube was made using 75-mm and 55-mm TLC (Ethicon Ltd., Somerville, NJ, USA) staplers, and the cervical anastomosis was performed in the left side of the neck with a 25-mm EEA stapler. The stomach was positioned in the posterior mediastinum. A pyloroplasty was routinely performed in all cases using finger disruption of the pylorus; the pylorus was pinched between the index finger and thumb until the pylorus ring was broken off.
After the operation, an esophagography was performed on postoperative day 7. Patients were allowed to take sips of water after confirming the absence of an anastomosis leak, and a full liquid diet was implemented on the following day. If the patient tolerated the liquid diet, the diet was then advanced to soft foods. We encouraged patients to ambulate soon after food was tolerated. All patients received metoclopramide HCl and H2 blockers postoperatively for 3 months, and a proton-pump inhibitor (PPI) was added if the patient experienced reflux symptoms.5
Clinical symptoms were assessed and documented at every outpatient department visit. The reflux symptoms were defined as nocturnal cough, pharyngeal regurgitation, cervical heartburn and lump sensation (globus pharyngis). Stricture-related symptoms were defined as dysphagia and odynophagia. For endoscopic evaluations, we used a gastroduodenoscope (GIF Q240 or H260; Olympus Optical Co., Ltd., Tokyo, Japan) with an outer diameter of 9.8 mm to examine the following parts of the upper gastrointestinal tract: the remnant esophagus, anastomotic site, gastric conduit and duodenum. Reflux esophagitis was classified into four grades, from A to D, according to the Los Angeles (LA) classification system.6 The definition of anastomotic stricture varies in the each literature. In this study, the authors defined an anastomotic stricture as a narrowing that prevented the passage of a standard gastroduodenoscope that had not been dilated previously, regardless of whether dysphagia was present, without evidence of malignancy. In cases of an anastomotic stricture, further endoscopic evaluation was performed using a pediatric gastroduodenoscope (GIF XP260; Olympus Optical Co., Ltd., Tokyo, Japan) with an outer diameter of 6.5 mm. If malignant anastomotic stricture was suspected, biopsy was performed.
The malignancies diagnosed on endoscopy were classified as one of the three following categories: a newly-developed malignancy, a recurrence at the anastomotic site or a regional lymph node recurrence with gastric conduit invasion. These endoscopic lesions were compared with findings on chest computed tomography (CT) and positron emission tomography (PET) scans. A differential diagnosis was made after a multidisciplinary discussion between the thoracic surgeons, the diagnostic radiologist, the thoracic oncologists and the endoscopists. Newly-developed malignancy was defined as new malignancy originated from the mucosa at the hypopharynx or remnant esophagus, at a distance from anastomotic sites. Recurrence at the anastomotic site was diagnosed when malignant lesions were detected at the anastomotic site. If a protruding lesion to the gastric conduit without mucosal invasion was detected on endoscopy, it was considered due to an external compression by regional lymph node recurrence with gastric conduit invasion. Regional lymph node recurrences were confirmed by CT and PET.
The chi-square test or Fisher's exact test was used to compare proportions between individual groups. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed by STATA11 software (StataCorp, 2005: Stata Statistical Software, Release 11, College Station, TX, USA).
From 2001 to 2008, 397 patients underwent esophagectomy and gastric reconstruction for esophageal squamous cell carcinoma at the National Cancer Center in South Korea. The surgical mortality rate was 12/397 (3.02%). Of 115 patients who died or had a recurrence within a year after surgery, 40 suffered cancer-related mortality, 29 suffered non-cancer-related mortality and 46 had a recurrence without related mortality. Sixty-nine patients were followed up at the other hospitals and were therefore unavailable for endoscopy. Thirty-nine patients underwent endoscopic follow-up within 6 months after the operation and were excluded from the study as a result. In total, 162 patients underwent endoscopy one year after their operation.
Of the 162 patients, 154 were male (95.1%). The mean age was 63.1±7.6 years. The pathologic diagnosis was squamous cell carcinoma in all cases. All patients received an R0 resection and the mean distance from the proximal margin to the cancer was 3.8±2.8 (0.2-11.5) cm. Cervical anastomosis was performed in 15 (9.3%) patients, and intrathoracic anastomosis was performed in 147 (90.7%) patients. The general characteristics of the patients are shown in Table 1. One hundred and two (62.9%) patients had no clinical symptoms. Twenty-two (13.6%) patients suffered from reflux symptoms (nocturnal cough in 10, regurgitation in 7, cervical heartburn in 3, lump sensation in 2). Forty-two (25.9%) patients suffered from stricture-related symptoms (dysphagia in 20 and odynophagia in 22). Four patients presented with both reflux and stenosis-related symptoms. Twenty-two patients with reflux symptoms were prescribed a PPI.
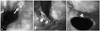
Normal endoscopic findings are shown in Fig. 1. However, 85 (52.5%) of the patients had abnormal findings. The types and incidences of these abnormal findings are described in Table 2. The mean anastomotic level was 20.7±1.5 cm from the upper incisors. The mean anastomotic level was 19.3±2.1 cm from the upper incisors for the cervical anastomosis group and 20.9±1.3 cm from the upper incisors in the intrathoracic anastomosis group.
Twelve patients (7.4%) had reflux esophagitis. According to LA classification, grade A esophagitis was found in six patients (3.7%), grade B in three (1.9%) and grade C in three (1.9%) (Fig. 2). No patients demonstrated severe reflux esophagitis of LA grade D. The reflux symptoms were not related to the presence of reflux esophagitis on endoscopic examinations (Fisher's exact test, p=0.680) (Table 3).
Thirty-seven patients (22.8%) had an anastomotic stricture. The presence of an anastomotic stricture was correlated with stricture-related symptoms (chi-square test, p<0.001) (Table 3). Eighteen symptomatic patients with anastomotic strictures underwent endoscopic- or fluoroscopic-guided balloon dilatation to relieve symptoms. Among the 22 patients who were symptomatic but had normal endoscopic examinations, 12 underwent outpatient department-based bougienage with a Hurst and Maloney bougie dilator (M-Flex™ Bleu Silicone Bougie, Medovations, Inc., Milwaukee, WI, USA) to resolve mild dysphagia. After fluoroscopic- or endoscopic-guided balloon dilatation, repeated outpatient department-based bougienage was performed to prevent recurrence of the stricture. Each patient's symptoms resolved after repeated balloon dilatation and outpatient bougienage. Of the 37 patients with anastomotic strictures, only one suffered a postoperative anastomotic leakage.
Two patients had recurrences at the anastomotic site, and four patients had a regional lymph node recurrence with gastric conduit invasion, as observed during endoscopy (Fig. 3A and B). In all six patients, these lesions were detected by CT or PET scan prior to endoscopic diagnosis, but they did not suffer from any clinical symptoms that implicated recurrence. Newly-developed malignancy in the hypopharynx was reported in one case, and newly-developed skip lesion was reported in the esophageal remnant in another (Fig. 3C). These were early lesions and were not detected by regular follow-up CT and PET scan without clinical symptoms related the newly-developed malignancy. Both patients underwent an additional operation and adjuvant radiation therapy. One patient underwent a partial hypopharyngectomy and primary repair with adjuvant radiation therapy, and he was still alive 26 months after the second operation. Another patient underwent a remnant esophagectomy, hypopharyngectomy and jejunal free graft with adjuvant radiotherapy, but he died 6 months later due to recurrence in the cervical lymph nodes and cancer progression.
Fourteen (8.6%) patients had erosive or superficial gastritis, and eight (4.9%) patients had gastric or duodenal ulcers in the gastric conduit.
The endoscopic findings were analyzed according to the level of anastomosis. Among 15 patients who underwent cervical anastomosis, there were 3 (20%) patients with anastomotic stricture and no patient with reflux esophagitis. Among 147 patients who underwent intrathoracic anastomosis, there were 34 (23.1%) patients with anastomotic stricture and 12 (8.2%) patients with reflux esophagitis. The level of anastomosis was not related to the development of anastomotic stricture (Fisher's exact test, p=1.0) or reflux esophagitis (Fisher's exact test, p=0.605).
After an esophagectomy and gastric reconstruction, clinicians face the challenge of managing various clinical symptoms during outpatient follow-up. To properly manage these symptoms, not only a patient's subjective complaints, but also objective diagnostic results are important.
Reflux esophagitis is one of the most common manifestations after esophagectomy and gastric reconstruction. Previous studies have reported that the incidence of reflux esophagitis ranges from 38-71%.7,8 The level of the anastomosis, the type of pyloroplasty and the route of reconstruction have been proposed as risk factors for reflux esophagitis, and the level of the anastomosis is regarded as a significant risk factor among these. Increased abdominal pressure pushes acid or bile contents into the remnant esophagus. Bemelman, et al.9 explained that, if anastomosis is performed at a lower level, the stomach is more affected by positive intra-abdominal pressure, and the incidence of reflux esophagitis is increased as a result. Compared with previous reports, the results of our study are remarkable in some aspects. First, the incidence of reflux esophagitis was 7.4% (12 patients), which is relatively low compared to previous reports. Second, severe reflux esophagitis, including LA grade D, was not reported. We think that these results can be attributed to our surgical policy of performing anastomosis as high as possible. In eastern countries, such as Korea, in contrast to western countries, most cases of esophageal cancer are squamous cell carcinoma. Esophageal squamous cell carcinoma frequently develops along the upper thoracic esophagus and commonly demonstrates skip metastases, with frequent involvement of recurrent laryngeal lymph nodes.10 Therefore, in our hospital, surgeons perform a cervical anastomosis or the highest level of intrathoracic anastomosis possible with a three-field dissection for upper thoracic esophageal cancer. Surgeons also perform the highest level of intrathoracic anastomosis with an extended two-field lymph node dissection, even for middle and lower esophageal cancers. As a result, the mean anastomotic level from the upper incisors was 19.3 cm in the cervical anastomosis group and 20.9 cm in the intrathoracic anastomosis group. According to D'Journo's11 report, their mean anastomotic level was 26.2±1.8 cm from the upper incisors for intrathoracic anastomosis and 22.2±2.3 cm from the upper incisors for cervical anastomosis. Our mean anastomotic level was relatively higher than what has previously been reported in western studies, which deal primarily with esophageal adenocarcinomas. In addition, the difference between the cervical anastomosis and the intrathoracic anastomosis in our study was only 1.6 cm. Considering the physiologic mechanisms mentioned above, a high anastomosis might explain the low incidence of both reflux esophagitis and severe reflux esophagitis in our series. Recently, Rice, et al.12 also reported that level of anastomosis was a risk factor for histopathologic changes due to reflux after esophagectomy and esophagogastric anastomosis.
The incidence of anastomotic stricture is relatively high, ranging between 10 and 56%.13 Generally, benign anastomotic strictures after esophagectomy and gastric reconstructions are thought to be associated with postoperative anastomotic leakage, conduit ischemia and a stapled (rather than a hand-sewn) anastomosis.14 Recently, some researchers have reported that benign anastomotic strictures can develop not only because of the previously proposed risk factors, but also as a consequence of severe gastroesophageal reflux of acid. Persistent exposure of the anastomotic site and remnant esophagus to gastric acid could result in mucosal erosion at the anastomotic site, causing a change in scarring during the healing process. Through repeated erosion and healing, scarring could progress to cause benign anastomotic strictures.3 Therefore, reducing the reflux of gastric acid can prevent not only reflux esophagitis but also benign anastomotic strictures. Johansson, et al.3 reported in a prospective study that prophylactic PPI treatment reduced the prevalence of benign anastomotic strictures after esophagectomy with gastric tube reconstruction. We advocated the administration of a PPI to reduce reflux of acid and prevent the formation of anastomotic stricture after an esophagectomy.
In this study, there were six cases of recurrences and two cases of newly-developed malignancies in the esophageal remnant or the hypopharynx. These patients did not show any clinical symptoms related to recurrence or newly-developed malignancies. Recurrence in mediastinal lymph nodes with distant metastases is frequently observed in advanced esophageal cancers after an operation and is usually detected by chest CT or PET scan. If these recurrent mediastinal lymph nodes invade the gastric conduit, a patient's quality of life is diminished due to dysphagia, and further treatment options would be limited. Therefore, the importance of endoscopic evaluation of recurrent esophageal cancer is to ascertain whether cancerous mediastinal lymph nodes invade the gastric conduit or not. Meanwhile, early-stage, newly-developed malignancies of the hypopharynx or the esophageal remnant may be detected on EGD, even if undetected on CT or PET scan. Clinicians must pay close attention not only to recurrence in mediastinal lymph nodes and at the anastomotic site, but also to newly-developed lesions at the esophagus or hypopharynx. If EGD is not performed, these lesions would be found at an advanced stage. Follow-up endoscopy may be helpful for detecting early-stage, curable, newly-developed malignancies.
Nocturnal cough, pharyngeal regurgitation, cervical heartburn and lump sensation are regarded as reflux symptoms. It has been reported that there is no correlation between clinical reflux symptoms and the presence of reflux esophagitis,2 and we have confirmed these results in our study. On the other hand, stricture-related symptoms, such as dysphagia and odynophagia, were statistically related to the presence of an anastomotic stricture. However, among the 37 patients who were diagnosed with anastomotic strictures on endoscopy, 19 (51.3%) had no symptoms of dysphagia. However, among the 37 patients who were diagnosed with anastomotic strictures on endoscopy, 19 had no symptoms of dysphagia. Approximately half of the patients with stricture-related symptoms had no anastomotic stricture. In fact, dysphagia can be anatomic and functional in origin. Some patients may have problems with swallowing caused not only by an anastomotic stricture, but by the weak propulsive waves of the esophageal remnant, which is denervated during manipulation prior to resection, and oropharyngeal dysmotility, which cannot be detected by endoscopy.15 Therefore, the presence of dysphagia alone does not equal to the presence of a stricture. The treatment of asymptomatic reflux esophagitis or anastomotic stricture is open for debate. In our center, we focus on the patient's symptoms. Symptomatic anastomotic strictures are treated with balloon dilatation. In patients with stricture-related symptoms but normal endoscopic exams, we sometimes perform outpatient bougienage. This treatment is already being performed at several centers, and is clinically practical and effective in resolving patient symptoms.16,17 In cases of asymptomatic reflux esophagitis, we first recommend life-style modifications such as small, frequent meals and sitting in an up-right position before prescribing a PPI, because long-term use of a PPI can have some adverse effects, such as infectious respiratory complications or nutritional deficiencies.18,19 If patients present with reflux symptoms without reflux esophagitis, then we prescribe a PPI to reduce the reflux symptoms. The response of PPI to reflux symptoms is not definite and about 60% (13 among 22 patients with reflux symptoms) of patients show a response. The management of reflux symptoms and reflux esophagitis after esophagectomy and gastric reconstruction warrants future study.
The fact that this study was retrospective is a limitation. Patients who died or had recurrence within one year after their operation or were in poor condition did not undergo follow-up endoscopy and were excluded from this analysis. As a result of these exclusions, the number of abnormal endoscopic findings may be underestimated. In addition, we did not perform biopsy of anastomotic sites and the remnant esophagus. Some articles demonstrated that endoscopic findings were not correlated with histopathologic results.11 Further study of clinical symptoms, endoscopic findings and histopathologic results is needed. However, the strengths of this study are not only the large number of patients compared to numbers in previous reports but also the consistency of our surgical methods and the regular intervals of the endoscopic follow-up. Also, we evaluated patients regardless of clinical symptoms.
In conclusion, half of the patients who underwent esophagectomy and gastric reconstruction demonstrated functional and oncological findings on follow-up endoscopic examination one year after surgery. With the exception of dysphagia and odynophagia, most clinical symptoms were not correlated with endoscopic findings. Furthermore, only endoscopy was able to detect newly-developed malignancies. For these reasons, routine endoscopic follow-up is a very useful tool for detecting latent functional and oncological lesions after esophagectomy, and clinicians must pay attention to both endoscopic findings and clinical symptoms.
Figures and Tables
Fig. 1
Normal findings at endoscopic follow-up one year after esophagectomy and gastric reconstruction for esophageal cancer. (A) Esophageal remnant. (B) Anastomotic site. (C) Gastric conduit.

Fig. 2
Endoscopic findings of reflux esophagitis according to the LA classification system. (A) LA grade A. (B) LA grade B. (C) LA grade C (arrows: areas of mucosal break). LA, Los Angeles.

Fig. 3
Oncologic lesions on endoscopy. (A) Recurrence at the anastomotic site. (B) Regional lymph node recurrence with gastric conduit invasion. (C) Newly-developed skip lesion in the esophageal remnant (arrow: mucosal lesion of recurrence at the anastomotic site, arrow head: mucosal lesion of a newly-developed skip lesion).
References
1. McLarty AJ, Deschamps C, Trastek VF, Allen MS, Pairolero PC, Harmsen WS. Esophageal resection for cancer of the esophagus: long-term function and quality of life. Ann Thorac Surg. 1997. 63:1568–1572.

2. Yuasa N, Sasaki E, Ikeyama T, Miyake H, Nimura Y. Acid and duodenogastroesophageal reflux after esophagectomy with gastric tube reconstruction. Am J Gastroenterol. 2005. 100:1021–1027.

3. Johansson J, Oberg S, Wenner J, Zilling T, Johnsson F, von Holstein CS, et al. Impact of proton pump inhibitors on benign anastomotic stricture formations after esophagectomy and gastric tube reconstruction: results from a randomized clinical trial. Ann Surg. 2009. 250:667–673.

4. Motoyama S, Saito R, Kitamura M, Suzuki H, Nakamura M, Okuyama M, et al. Prospective endoscopic follow-up results of reconstructed gastric tube. Hepatogastroenterology. 2003. 50:666–669.
5. Lee HS, Kim MS, Lee JM, Kim SK, Kang KW, Zo JI. Intrathoracic gastric emptying of solid food after esophagectomy for esophageal cancer. Ann Thorac Surg. 2005. 80:443–447.

6. Armstrong D. Endoscopic evaluation of gastro-esophageal reflux disease. Yale J Biol Med. 1999. 72:93–100.
7. Gutschow C, Collard JM, Romagnoli R, Salizzoni M, Hölscher A. Denervated stomach as an esophageal substitute recovers intraluminal acidity with time. Ann Surg. 2001. 233:509–514.

8. Shibuya S, Fukudo S, Shineha R, Miyazaki S, Miyata G, Sugawara K, et al. High incidence of reflux esophagitis observed by routine endoscopic examination after gastric pull-up esophagectomy. World J Surg. 2003. 27:580–583.

9. Bemelman WA, Verburg J, Brummelkamp WH, Klopper PJ. A physical model of the intrathoracic stomach. Am J Physiol. 1988. 254(2 Pt 1):G168–G175.

10. Matsubara T, Ueda M, Kaisaki S, Kuroda J, Uchida C, Kokudo N, et al. Localization of initial lymph node metastasis from carcinoma of the thoracic esophagus. Cancer. 2000. 89:1869–1873.

11. D'Journo XB, Martin J, Rakovich G, Brigand C, Gaboury L, Ferraro P, et al. Mucosal damage in the esophageal remnant after esophagectomy and gastric transposition. Ann Surg. 2009. 249:262–268.
12. Rice TW, Goldblum JR, Rybicki LA, Rajeswaran J, Murthy SC, Mason DP, et al. Fate of the esophagogastric anastomosis. J Thorac Cardiovasc Surg. 2011. 141:875–880.

13. Orringer MB, Marshall B, Iannettoni MD. Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg. 2000. 119:277–288.

14. Williams VA, Watson TJ, Zhovtis S, Gellersen O, Raymond D, Jones C, et al. Endoscopic and symptomatic assessment of anastomotic strictures following esophagectomy and cervical esophagogastrostomy. Surg Endosc. 2008. 22:1470–1476.

15. Kim HK, Choi YH, Shim JH, Cho YH, Baek MJ, Sohn YS, et al. Endoscopic evaluation of the quality of the anastomosis after esophagectomy with gastric tube reconstruction. World J Surg. 2008. 32:2010–2014.

16. Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999. 230:392–400.

17. Orringer MB, Lemmer JH. Early dilation in the treatment of esophageal disruption. Ann Thorac Surg. 1986. 42:536–539.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download