Abstract
Purpose
Whether addition of cilostazol is superior to increasing dose of clopidogrel in patients with hyporesponsiveness to chronic clopidogrel therapy is unknown.
Materials and Methods
We studied 73 patients with hyporesponsiveness to clopidogrel on standard dual antiplatelet therapy for more than 2 weeks. Clopidogrel hyporesponsiveness was defined as percent inhibition of P2Y12 reaction units (PRU) <30% on VerifyNow P2Y12 assay. Patients were randomly assigned to increased dose of clopidogrel (aspirin 100 mg+clopidogrel 150 mg daily: group A, n=38) or to receiving additional cilostazol (aspirin 100 mg+clopidogrel 75 mg+cilostazol 100 mg bid daily: group B, n=35).
Results
Baseline percent inhibition of PRU and PRU was similar between 2 groups (13.0±10.2% versus 11.8±9.7%, p=0.61, and 286.3±54.7 versus 295.7±53.7, p=0.44, respectively). At follow-up, percent inhibition of PRU was higher and PRU was lower significantly in group B than in group A (38.5±17.9% versus 28.3±16.6%, p=0.02, and 207.3±68.2 versus 241.3±76.7, p=0.050, respectively). Among those still showing hyporesponsiveness to clopidogrel at follow-up (21 patients in group A, 10 patients in group B), 12 patients completed further crossover study. Compared to the baseline, magnitude of change in percent inhibition of PRU and PRU showed an improved tendency after the crossover (from 2.7±8.7% to 15.8±18.4%, p=0.08, and from -18.6±58.0 to -61.9±84.3, p=0.08).
Response to clopidogrel shows wide individual variability, and substantial portion of patients have hyporesponsiveness or resistance to clopidogrel.1 Increase of clopidogrel dose or addition of cilostazol has been reported to enhance the responsiveness of platelets to clopidogrel.2,3 Currently, there are several reports showing the superiority of addition of cilostazol over higher maintenance dose of clopidogrel to improve the responsiveness to clopidogrel.4,5 In these studies, however, the response of platelets was assessed just before the coronary intervention after 300 mg or 600 mg loading dose of clopidogrel. Meanwhile, there are limited data assessing the platelet activity in a steady state of clopidogrel after a substantial period of usual maintenance dose. Since the stent thrombosis of drug-eluting stent (DES) is not a problem confined to acute coronary syndrome, it is a notable issue to find more efficient regimen in the hypo-responsive group in a chronic stable condition after DES implantation.
The primary aim of this study is to investigate whether the addition of cilostazol is superior to increasing dose of clopidogrel in the patients with hyporesponsiveness to clopidogrel in spite of standard dual antiplatelet therapy for more than 2 weeks. Additionally, we performed another 4 weeks of crossover study targeting the patients with hyporesponsiveness to clopidogrel after 4 weeks of initial intensified antiplatelet therapy.
The study was a single-center, prospective, open-labeled, randomized platelet function study to compare the effects of adjunctive cilostazol versus high maintenance dose of clopidogrel in the patients with hyporesponsiveness to clopidogrel. Patients who showed hyporesponsiveness to clopidogrel under the standard dual antiplatelet therapy (aspirin 100 mg/day+clopidogrel 75 mg/day) for more than 2 weeks after DES implantation was included. Exclusion criteria were as follows: hypersensitivity to aspirin, clopidogrel, or cilostazol; congestive heart failure; acute coronary syndrome within 2 weeks; cerebrovascular event within 3 months; major bleeding within 3 months; bleeding diathesis; thrombocytopenia (<100000/uL); hematocrit <30%; use of glycoprotein IIb/IIIa receptor antagonist within 1 month; concomitant use of warfarin; renal dysfunction (serum creatinine >2 mg/dL); liver disease (serum bilirubin >2 mg/dL).
Patients meeting inclusion and exclusion criteria were randomly assigned to increased dose of clopidogrel (aspirin 100 mg+clopidogrel 150 mg daily: group A), or to receiving additional cilostazol (aspirin 100 mg+clopidogrel 75 mg daily+cilostazol 100 mg bid daily: group B). Patients were randomly assigned in a ratio of 1 : 1, with the use of balanced computer-generated blocks of four, to either group A or group B. The median interval at the time of randomization after DES implantation was similar in both groups, 30 (29-33) days in group A and 30 (24-32) days in group B (p=0.324). Platelet function test was performed 4 weeks after randomization. Each patient's compliance was monitored by counting the number of pills remaining at follow-up time. Patients still showing hyporesponsiveness to clopidogrel after 4 weeks of randomization were asked to participate in the crossover study. Patients who agreed to be enrolled, received crossover treatment, and follow-up platelet function test was repeated 4 weeks after the crossover. There was no washout phase between 2 treatment periods.
Responsiveness to clopidogrel was measured using the VerifyNow P2Y12 assay (Accumetrics Inc., San Diego, CA, USA). The VerifyNow P2Y12 assay is a rapid whole blood point-of-care test for measuring the effects of clopidogrel on the P2Y12 receptor.
The VerifyNow-P2Y12 system contains 20 µmol of adenosine diphosphate (ADP) to activate P2Y1 and P2Y12 receptors and 22 µmol of prostaglandin E1 to reduce nonspecific contribution of the P2Y1 receptor. The assay measures the change in the optical signal caused by ADP-induced platelet aggregation, using cartridges containing fibrinogen coated beads. The results are expressed in P2Y12 reaction units (PRU). Percent inhibition of ADP-induced platelet aggregation is also calculated as follows: [1-(PRU after clopidogrel/PRU at baseline)]×100. The PRU at baseline is obtained in the thrombin receptor activating peptide channel and serves as an estimate of the baseline platelet function independent of P2Y12 inhibition or without having a value prior to clopidogrel. Hyporesponsiveness to clopidogrel was defined as percent inhibition of ADP-induced platelet aggregation <30%.6,7
The primary endpoint was percent inhibition of PRU at follow-up of 4 weeks after randomization. The secondary end points included PRU at follow-up of 4 weeks after randomization, change in percent inhibition of PRU and PRU.
The primary endpoints of group A and group B were assumed to be 30±15% and 40±15%, respectively, which are approximation between the values in two studies. In ACCEL-RESISTANCE study, percent inhibition of PRU was 23.1±29.9% vs. 39.6±24.1% in each high maintenance dose of clopidogrel and triple antiplatelet group.4 And ACCEL-AMI study showed similar pattern with percent inhibition of 30.7±27.5% vs. 43.0±24.4%, respectively. Considering α-error as 0.05 and β-error as 0.80, the required sample size was 36. Assuming that drop-out rate would be 10%, we included 40 patients in each group.
Data are expressed as mean±SD or frequencies. Categorical variables were compared by χ2 tests or Fisher's exact tests as seems to be appropriate. Comparison of the continuous variables was performed by independent t-test or Mann-Whitney U test. Baseline and follow-up test results were compared with paired t-test or Wilcoxon signed ranks test where appears to be appropriate. p values are two-tailed and p<0.05 was considered significant. PASW® Statistics 18 (SPSS Inc., Chicago, IL, USA) was used in the statistical analysis.
The institutional review board of Samsung Medical Center approved this study, and all subjects gave their informed consent to participation. This study is registered with ClinicalTrials.gov, number NCT00620646.
From February 2008 to November 2008, a prospective but nonconsecutive series of 318 patients on standard dual antiplatelet therapy for more than 2 weeks underwent VerifyNow P2Y12 assay to screen the responsiveness to clopidogrel. Of these, 80 patients met inclusion and exclusion criteria, but 1 patient refused to participate in this study. Finally, 79 patients were enrolled: 39 patients were randomly assigned to increased dose of clopidogrel (group A) and 40 to receiving additional cilostazol (group B). In group A, 1 patient had diarrhea and stopped the medication earlier than planned. In group B, 5 patients needed to withdraw the planned protocol due to severe headache. As a result, total 38 patients in group A and 35 patients in group B completed the trial. The patient flow is summarized in Fig. 1. Baseline characteristics including hemoglobin level and platelet count did not differ significantly between group A and group B. Medications which were taken at baseline were not significantly different, either (Table 1). During follow-up period, there was no minor/major bleeding, or adverse cardiovascular events in both groups.
Baseline percent inhibition of PRU and PRU was similar in both group B and group A (13.0±10.2% versus 11.8±9.7%, p=0.61, and 286.3±54.7 versus 295.7±53.7, p=0.44, consecutively). At follow-up, percent inhibition of PRU was significantly greater in group B than in group A (38.5±17.9% versus 28.3±16.6%, p=0.02). The level of PRU was also statistically lower in group B at 4 week-follow-up (207.3±68.2 versus 241.3±76.7, p=0.050). Similarly, the change in percent inhibition of PRU was statistically much larger in group B (24.9±17.0% versus 16.4±17.2%, p=0.04). Although there was no statistically significant difference in the change of PRU between the two groups, group B showed a tendency of larger change in PRU (-78.1±65.1 versus -54.2±67.6, p=0.13) (Fig. 2).
Twenty-one among 38 patients (55.3%) in group A, and 10 among 35 patients (28.6%) in the group B showed follow-up percent inhibition of PRU less than 30% at 4 weeks after the 1st randomization. The percentage of sustained hyporesponsiveness to clopidogrel was significantly lower in group B than in group A (p=0.02). Among them, 13 patients agreed to be continually enrolled in the crossover study. They included 10 patients from group A and 3 patients from group B. One patient who switched the regimen from A to B had to cease the medication earlier due to severe headache. Therefore, 12 patients completed the crossover study. At the second follow-up, total 5 patients achieved optimal response to clopidogrel with the percent inhibition of PRU over 30% (Fig. 3A). They included 1 patient changing the regimen from additive cilostazol to increased dose of clopidogrel and 4 patients from increased dose of clopidogrel to additive cilostazol. The number of patients enrolled in the crossover study was small to be statistically analyzed. However, compared to the baseline, magnitude of change in percent inhibition of PRU and PRU showed an improved tendency after the crossover (from 2.7±8.7% to 15.8±18.4%, p=0.08, and from -18.6±58.0 to -61.9±84.3, p=0.08) (Fig. 3B and C).
This study demonstrated that adjunctive cilostazol was superior to higher maintenance dose of clopidogrel in improving responsiveness to clopidogrel in hypo-responsive patients to chronic dual antiplatelet therapy after DES. In patients who remained persistently hyporesponsive to clopidogrel in spite of intensified antiplatelet therapy, crossover treatment seems to further improve platelet inhibition.
One of the problems encountered with clopidogrel is that response to clopidogrel shows wide individual variability, and a substantial portion of patients have hyporesponsiveness or resistance to clopidogrel.1 Since hypo-responsiveness to clopidogrel has been reported to be associated with adverse clinical outcomes,8,9 finding more effective regimen in the patients with hyporesponsiveness to clopidogrel has evoked great interests. Increase of clopidogrel dose or addition of cilostazol to the standard dual anti-platelet therapy was suggested as another option to solve this problem.2,3,10-12 The ACCEL-RESISTANCE and ACCEL-AMI study compared these two regimens and demonstrated that platelet inhibition of adjunctive cilostazol was more intense than 150 mg maintenance dose of clopidogrel.4,5 However, baseline platelet function test was performed immediately before PCI in the above study. Therefore, they could not reflect the steady state obtained only after a substantial period of clopidogrel treatment.13
As a phosphodiesterase III inhibitor, cilostazol acts on a pathway different from clopidogrel, one of the P2Y12 receptor inhibitor.14 Cilostazol inhibits phophodiesterase activity, suppresses cyclic adenosine monophosphate degradation, and activates vasodilator-stimulated phosphoprotein. Hence, cilostazol acts on the pathway which belongs to the downstream of clopidogrel action without passing through P2Y12 receptor.15,16 Moreover, cilostazol is metabolized mainly by cytochrome P450 (CYP) 3A4 pathway and, to a lesser extent, by CYP2C19, the major metabolizing pathway of clopidogrel.16 Those pharmacologic aspects of cilostazol can be a good reason of why cilostazol is superior to higher maintenance dose of clopidogrel.
Meanwhile, the side effect of cilostazol should not be ignored before conceding triple antiplatelet therapy as superior one. In our study, 5 patients among 40 patients in group B and 1 patient among 3 patients in group B after crossover had to halt cilostazol due to severe headache. Gastrointestinal irritation and headache are very common adverse events of cilostazol.16 Previous study reported that the discontinuation rate due to adverse events in triple group was 15.2%,3 thus further implicating the needs of individually-tailored prescription, weighing the feasibility of long-term medication and the effectiveness of antiplatelet-regimen, when treating poor responders to clopidogrel.
In patients with persistent hyporesponsiveness to clopidogrel in spite of intensified therapy, crossover treatment further improved platelet inhibition. Patients remaining hyporesponsive to clopidogrel on the alternative antiplatelet regimen could have a chance to increase the platelet inhibition by changing the strategy to other options. Although the number of enrolled patients in the crossover study was very small and statistical significance was not observed, the tendency in our study suggested the need of further investigation.
Our study has several limitations. First, it included a relatively small number of patients, although we beforehand calculated the sample size to have a statistical power of widely accepted level. Second, we measured platelet function or clopidogrel response using only VerifyNow assay instead of the conventional light transmittance aggregometry (LTA). However, the result of VerifyNow has been shown to closely correlate with other platelet function tests including LTA.17 Furthermore, there is a report showing clinical correlation with thrombotic events after DES implantation using VerifyNow assay.9,18 We used percent inhibition of PRU <30% as the definition of hyporesponsiveness to clopidogrel. However, it is criteria less strict than previous reports which showed the clinical outcome.6 Consequently, there remains a possibility to over-estimate the poor-responder group. Meanwhile, a recent paper indicated superiority of cilostazol over high maintenance dose of clopidogrel, especially in CYP2C19 loss-of-function variants,19 and an another recent study reported cut-off level of 26.5% inhibition for the identification of CYP2C19 reduced-function allele carrier.7 Therefore, a 30% as cut-off value might not necessarily over-estimate the number of hyporesponsive patients to clopidogrel. Third, the number of patients enrolled in the crossover study was small to be statistically optimally analyzed. Finally, we did not include wash-out phase before the crossover study. There is a chance that there could have been some carry-over effects in the results of this crossover study. However, the previous antiplatelet protocol was less likely to influence the results of the platelet function test after 4 weeks of changing the regimen, considering the half-life of clopidogrel and cilostazol.
In conclusion, adjunctive cilostazol improved clopidogrel responsiveness better than higher maintenance dose of clopidogrel in hyporesponsive patients with chronic clopidogrel therapy.
Figures and Tables
 | Fig. 2Percent inhibition of PRU and PRU at baseline and follow-up. (A) At baseline, group A and B showed no statistical difference in percent inhibition of PRU. After 4 weeks of randomization, group B showed significantly higher value of percent inhibition of PRU. (B) Group A and B initially presented no statistical difference in PRU. After 4 weeks of randomization, group B showed significantly lower value of PRU. (C) In the difference in percent inhibition, group B showed statistically larger change compared to group A after 4 weeks of antiplatelet regimens. (D) Group B showed the similar tendency of the larger change in PRU compared to group A after 4 weeks of antiplatelet regimens. PRU, P2Y12 reaction units. |
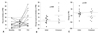 | Fig. 3Data of the patients enrolled the followed crossover study. (A) Among the 12 patients enrolled crossover study, total 5 patients overcame clopidogrel hypo-responsiveness. (B) In change in percent inhibition of PRU from baseline, the crossover data showed a tendency of overall improved platelet inhibition. (C) In change in PRU from baseline, the patients also showed the larger degree of platelet inhibition in trend after the crossover. PRU, P2Y12 reaction units. |
References
1. Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005. 45:246–251.

2. Angiolillo DJ, Capranzano P, Goto S, Aslam M, Desai B, Charlton RK, et al. A randomized study assessing the impact of cilostazol on platelet function profiles in patients with diabetes mellitus and coronary artery disease on dual antiplatelet therapy: results of the OPTIMUS-2 study. Eur Heart J. 2008. 29:2202–2211.

3. Lee SW, Park SW, Kim YH, Yun SC, Park DW, Lee CW, et al. Comparison of triple versus dual antiplatelet therapy after drug-eluting stent implantation (from the DECLARE-Long trial). Am J Cardiol. 2007. 100:1103–1108.

4. Jeong YH, Lee SW, Choi BR, Kim IS, Seo MK, Kwak CH, et al. Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity: results of the ACCEL-RESISTANCE (Adjunctive Cilostazol Versus High Maintenance Dose Clopidogrel in Patients With Clopidogrel Resistance) randomized study. J Am Coll Cardiol. 2009. 53:1101–1109.

5. Jeong YH, Hwang JY, Kim IS, Park Y, Hwang SJ, Lee SW, et al. Adding cilostazol to dual antiplatelet therapy achieves greater platelet inhibition than high maintenance dose clopidogrel in patients with acute myocardial infarction: results of the adjunctive cilostazol versus high maintenance dose clopidogrel in patients with AMI (ACCEL-AMI) study. Circ Cardiovasc Interv. 2010. 3:17–26.

6. Lee K, Lee SW, Lee JW, Kim SY, Youn YJ, Ahn MS, et al. The significance of clopidogrel low-responsiveness on stent thrombosis and cardiac death assessed by the verifynow p(2)y(12) assay in patients with acute coronary syndrome within 6 months after drug-eluting stent implantation. Korean Circ J. 2009. 39:512–518.

7. Ono T, Kaikita K, Hokimoto S, Iwashita S, Yamamoto K, Miyazaki Y, et al. Determination of cut-off levels for on-clopidogrel platelet aggregation based on functional CYP2C19 gene variants in patients undergoing elective percutaneous coronary intervention. Thromb Res. 2011. 128:e130–e136.

8. Buonamici P, Marcucci R, Migliorini A, Gensini GF, Santini A, Paniccia R, et al. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol. 2007. 49:2312–2317.

9. Price MJ, Endemann S, Gollapudi RR, Valencia R, Stinis CT, Levisay JP, et al. Prognostic significance of post-clopidogrel platelet reactivity assessed by a point-of-care assay on thrombotic events after drug-eluting stent implantation. Eur Heart J. 2008. 29:992–1000.

10. Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation. 2007. 115:708–716.

11. Kastrati A, von Beckerath N, Joost A, Pogatsa-Murray G, Gorchakova O, Schömig A. Loading with 600 mg clopidogrel in patients with coronary artery disease with and without chronic clopidogrel therapy. Circulation. 2004. 110:1916–1919.

12. von Beckerath N, Kastrati A, Wieczorek A, Pogatsa-Murray G, Sibbing D, Graf I, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J. 2007. 28:1814–1819.

13. Wallentin L, Varenhorst C, James S, Erlinge D, Braun OO, Jakubowski JA, et al. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J. 2008. 29:21–30.

14. Mullangi R, Srinivas NR. Clopidogrel: review of bioanalytical methods, pharmacokinetics/pharmacodynamics, and update on recent trends in drug-drug interaction studies. Biomed Chromatogr. 2009. 23:26–41.

15. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007. 49:1505–1516.
16. Dindyal S, Kyriakides C. A review of cilostazol, a phosphodiesterase inhibitor, and its role in preventing both coronary and peripheral arterial restenosis following endovascular therapy. Recent Pat Cardiovasc Drug Discov. 2009. 4:6–14.

17. von Beckerath N, Sibbing D, Jawansky S, Braun S, Morath T, Vogt W, et al. Assessment of platelet response to clopidogrel with multiple electrode aggregometry, the VerifyNow P2Y12 analyzer and platelet Vasodilator-Stimulated Phosphoprotein flow cytometry. Blood Coagul Fibrinolysis. 2010. 21:46–52.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download