Abstract
Purpose
The aim of this study was to demonstrate the early effects of statin treatment on plaque composition according to plaque stability on Intravascular Ultrasound-Virtual Histology at 6 months after a coronary event. Previous trials have demonstrated that lipid lowering therapy with statins decreases plaque volume and increases plaque echogenicity in patients with coronary artery disease.
Materials and Methods
Fifty-four patients (54 lesions) with acute coronary syndrome were prospectively enrolled. We classified and analyzed the target plaques into two types according to plaque stability: thin-cap fibroatheroma (TCFA, n=14) and non-TCFA (n=40). The primary end point was change in percent necrotic core in the 10-mm subsegment with the most disease.
Results
After 6 months of statin therapy, no change was demonstrated in the mean percentage of necrotic core (18.7±8.5% to 20.0±11.0%, p=0.38). There was a significant reduction in necrotic core percentage in patients with TCFA (21.3±7.2% to 14.4±8.9%, p=0.017), but not in patients with non-TCFA. Moreover, change in percent necrotic core was significantly correlated with change in high-sensitivity C-reactive protein levels (r=0.4, p=0.003). Changes in low-density lipoprotein cholesterol levels and lipid core percentage demonstrated no significant associations.
Histopathologic data indicate that plaque composition is a major determinant of the tendency of atherosclerotic lesions to provoke clinical events. In particular, thin-cap fibroatheroma (TCFA) plaques with macrophage and large necrotic cores are at high risk for rupture and result in epicardial coronary occlusion.1,2 Recent data suggest that spectral analysis of intravascular radiofrequency ultrasound, known as Intravascular Ultrasound-Virtual Histology (IVUS-VH), offers an opportunity to simultaneously assess the morphological and histopathological characteristics of plaque. This ability allows IVUS-VH to define TCFA plaques in vivo.3-5
Numerous clinical trials have shown that lipid lowering therapy with statins reduces cardiovascular morbidity and mortality, with significant effects evident only after 12 to 24 months of treatment. Although these trials excluded patients with recent unstable angina or acute myocardial infarction (MI),6,7 patients experience the highest rates of death and recurrent cardiovascular ischemic events during the early period after an acute coronary syndrome (ACS).8 Therefore, strategies to stabilize vulnerable plaques during the early high-risk period are of paramount importance. Recently, early statin treatment in patients with ACS showed a significant advantage at short-term follow-up.9,10 Of note, early aggressive lipid-lowering by atorvastatin induced a significant reduction in coronary plaque volume, assessed by volumetric IVUS analysis at 6 months after the onset of ACS.11 This early benefit in ACS might be explained by the difference in target plaque characteristics between ACS and stable coronary artery disease, and is expected to be more pronounced in patients with vulnerable plaque. We therefore hypothesized that target plaques according to plaque stability may exhibit different susceptibilities to statin therapy during the early period of ACS; specifically, TCFA, representative of vulnerable plaque, would demonstrate earlier and greater reduction of lipid core compared with non-TCFA. To test this hypothesis, we prospectively examined IVUS-VH in our patients with ACS at baseline and after 6 months on statin therapy. Because this may also contribute to demonstrating the early benefits of statin following ACS in vivo, the roles of lipid profile and systemic levels of C-reactive protein (CRP) were also investigated.
This prospective, single-center study was designed to assess the effect of 6 months of treatment with statin on induction of plaque composition change in non-PCI sites of the culprit vessel by serial volumetric IVUS-VH analysis. Patients received statin treatment immediately after PCI. Use of lipid-lowering medication for >3 months within the previous 12 months was not allowed. Patients who received lipid-lowering therapy in the 4 weeks prior to enrollment were also excluded. Patients aged 18 to 75 years were eligible for inclusion in the present study if they had been recommended for an intracoronary revascularization procedure and had successfully undergone intracoronary intervention. The target plaque was qualified for the IVUS study if it had not been influenced by any previous therapeutic intervention, and if the diameter of the stenosis was <50% on quantitative coronary angiography. The plaque had to be >10 mm proximal to the acute intervention site. Another coronary artery was imaged if there was no plaque visible in the intervened vessel.3 Bifurcation lesions, lesions with severe angulation, heavily calcified lesions, and lesions with poor image quality were excluded from the present study. ACS was defined as unstable angina, non-ST-segment elevation MI or ST-segment elevation MI. The present study was approved by the hospital's Ethics Committee, and written informed consent was obtained from all patients.
Details regarding the validation of the technique, on explanted human coronary segments, have previously been reported.3 Briefly, IVUS-VH (Volcano Corp., Rancho Cordova, CA, USA) uses spectral analysis of IVUS radiofrequency data to construct tissue maps that classify plaque into four major components. In preliminary in vitro studies, four histological plaque components (fibrous, fibro-lipid, necrotic core, and calcium) were correlated with a specific spectrum of the radiofrequency signal. These different plaque components were assigned color codes. Calcified, fibrous, fibrolipidic, and necrotic core regions were labeled white, green, greenish-yellow, and red, respectively. IVUS-VH data were acquired after intracoronary administration of nitrates using a continuous pullback (0.5 mm/s) with commercially available mechanical sector scanners (2.9 Fr Eagle Eye 20-MHz catheter, Volcano Corp., Rancho Cordova, CA, USA) by a dedicated IVUS-VH console (Volcano Corp., Rancho Cordova, CA, USA). The IVUS-VH data were stored on a CD-ROM/DVD and sent to the imaging core lab for offline analysis (Asan Medical Center, Seoul, Korea). Manual contour detection of both the lumen and the media-adventitia interface was performed. IVUS-VH analyses were reported in absolute amounts and as percentages (relative amounts) of each plaque. The target segment was selected and determined with a reproducible index side branch. Geometrical and compositional data were obtained for each cross-sectional area (CSA), and an average was calculated for the most diseased 10-mm subsegments. Baseline and follow-up IVUS-VH images (6 months) were reviewed side by side on a display. The primary end point of this study was change in percent necrotic core (baseline minus follow-up).
Two experienced, independent IVUS analysts defined IVUS-derived TCFA as a lesion fulfilling the following criteria in at least three consecutive frames: 1) necrotic core ≥10% without evident overlying fibrous tissue and 2) percent atheroma volume ≥40%.
To assess the reproducibility of VH measurement, baseline images of 10 cases were randomly selected. The interobserver correlation coefficient for percent necrotic core was 0.994 and the percentage of errors was 0.36±0.75%. There was little intra-observer or inter-observer disagreement in the diagnosis of VH-derived TCFA (intra-observer; κ=0.83, 95% CI, 0.74-0.92, inter-observer; κ=0.80, 95% CI, 0.70-0.89).
Gray-scale IVUS measurements of external elastic membrane, plaque and media and lumen CSAs, and plaque burden (plaque and media divided by external elastic membrane) were performed for every recorded frame; volumetric data were generated by software using Simpson's method.
CRP levels were measured in serum using a commercially available kit (N High Sensitivity CRP, Dade Behring, Marburg, Germany). Plasma concentrations of total cholesterol, high-density lipoprotein cholesterol, and triglycerides were measured at our clinical laboratory. The Friedewald formula was used to derive low-density lipoprotein cholesterol (LDL-C) levels. Changes of lipid and inflammatory parameters were calculated as the difference between baseline and follow-up values.
Simple descriptive statistics were used to summarize the data. Categorical variables are described using frequencies and percentages. For continuous variables with a normal distribution, means±SD are reported. For CRP levels, which were not normally distributed, median and inter-quartile ranges (IQR) are reported. Correlations between variables are described with the use of Spearman rank-correlation coefficients. To assess inter- and intra-observer variability, results were compared using the κ-test of concordance for the categorical data. p-values <0.05 were considered statistically significant.
Between January 2007 and May 2008, 64 patients with ACS were enrolled in this study. Six patients were withdrawn because they refused follow-up angiography. Four patients did not take statins during the follow-up period. Thus, a total of 54 patients (54 lesions) completed protocol. The mean patient age was 59±10 years; 38 patients (70%) were men; and 50 patients were diagnosed with unstable angina. We classified and analyzed the target plaques into two types according to plaque stability: TCFA (n=14) and non-TCFA (n=40). Demographic characteristics and baseline medications were similar in both groups (Table 1), with the exception of a significant difference in median CRP levels: 2.53 mg/L (IQR; 0.95 mg/L to 8.40 mg/L) in patients with TCFA versus 1.02 mg/L (IQR; 0.42 mg/L to 2.00 mg/L) in patients with non-TCFA (p=0.02).
Patients were treated with various statins as follows: 40 (74%) atorvastatin, 8 (15%) simvastatin, and 6 (11%) rosuvastatin. Baseline and follow-up laboratory characteristics are summarized in Table 2. Statin treatment resulted in a 44% reduction in LDL-C level to a mean of 67.3 mg/dL; CRP level decreased by 47% to a mean of 1.69 mg/L.
Table 3 shows the findings of IVUS-VH measurements at baseline and follow-up. On the analysis of primary efficacy parameters in the entire patient population (n=54), no change in percent necrotic core (18.7±8.5% to 20.0±11.0%, p=0.38) was demonstrated in the most diseased 10-mm subsegments. Similarly, there was no change in percent necrotic core at the minimum lumen site (19.8±10.0% to 20.3±11.8%, p=0.76) and largest necrotic core site (26.6±9.6% to 29.5±12.5%, p=0.1). When we performed the analysis according to plaque stability, however, there was a significant reduction in necrotic core percentage (21.3±7.2% to 14.4± 8.9%, p=0.017) and absolute volume (12.5±6.7 mm3 to 8.1±8.4 mm3, p=0.018) in patients with TCFA. On the other hand, no changes in absolute volume and necrotic core percentage were observed in patients with non-TCFA (Fig. 1). Among 14 patients with TCFA, 13 patients showed a reduction in percent necrotic core at 6 months. However, 18 patients (out of 40) with non-TCFA showed a decrease in necrotic core after statin therapy. The analysis of change in each plaque component in 14 patients with TCFA showed that the percentage of fibrotic plaque was increased (60.6±9.6% to 66.4±9.9%, p=0.14), although statistical significance was not reached (Fig. 2). Fig. 3 shows the IVUS-VH images of a representative patient for each type of plaque. Baseline and follow-up IVUS-VH images are presented side by side. Marked reduction in the necrotic core is clearly observed in a patient with TCFA only after 6 months of pharmacological intervention, whereas an increase in the necrotic core is observed in a patient with non-TCFA. Table 4 shows the baseline and 6-month follow-up data of volumetric gray-scale IVUS analysis in the target plaque. Plaque volume was significantly reduced (2.85±10.99% decrease; p=0.03 for baseline versus follow-up), as well were lumen and vessel volume.
There was no significant correlation between LDL-C level reduction and decrease in CRP (r=0.2, p=0.14). Change in percent necrotic core showed a significant correlation with change in CRP level (r=0.4, p=0.003). In contrast, changes in LDL-C level and lipid core demonstrated no significant associations (Fig. 4).
Our study investigated whether early statin therapy in patients with vulnerable plaque compared with stable plaque reduces necrotic core assessed by IVUS-VH at 6 months. The results of this study clearly showed a reduction of lipid core in only patients with TCFA, which suggests that plaque stabilization following statin therapy might occur earlier in vulnerable plaque than in stable plaque. In addition, change in necrotic core showed a significant correlation with change in CRP level. This is the first report to clarify the relationship between plaque stability and statin response, particularly during the early period of ACS, and to further examine the influence of a systemic inflammatory marker on plaque composition in vivo.
Goldstein, et al.12 suggested that plaque instability might reflect a "pan-coronary" process. Their concept of multifocal plaque instability was supported by angiographic natural history studies in patients with ACS, in whom rapid progression not only occurred in culprit lesions, but also in nonculprit lesions. In a serial angiographic evaluation of our recent data, multiple complex plaques in non-culprit lesions were also identified in 27% of acute ST-segment elevation MI.13 It seems likely that overall coronary instability is responsible for the frequent recurrence rate after acute treatment of culprit lesions in ACS. In fact, it is within the early period after an ACS that patients experience the highest rate of death and recurrent ischemic events.8 Recently, the MIRACL and PROVE-IT trials indicated that early intensive statin initiation during the acute phase of ACS reduces the risk of recurrent ischemic events.9,10 However, there is little scientific basis for recommending early statin initiation to reduce recurrent ischemic events.14 Our study may provide insight into the early benefit of statin following ACS. As a matter of fact, we substantiated our speculation that the benefit of early statin therapy could be more pronounced in patients with vulnerable plaque than in those with stable plaque. To confirm our data, determination of whether or not percent necrotic core in non-TCFA is decreased in the long-term is needed.
In contrast to the observation presented here, recent data provide conflicting evidence that early statin therapy does not reduce the incidence of major cardiovascular events.15 However, there were several differences among previous studies, including population selection, baseline risk profile, and statin initiation time. In addition, some of the benefits of statin treatment in the early period after ACS may only become manifested or evident in long-term follow-up, because it is likely that the beneficial effects of statins are cumulative. Further studies are warranted.
Parallel to clinical outcomes trials, imaging studies, in particular IVUS-based studies, have substantially contributed to understanding the benefits of lipid lowering therapies in coronary artery disease. In fact, these studies have demonstrated slowing of atherosclerosis progression or regression as a surrogate marker of clinical outcomes after statin therapy.16-18 However, these modalities may not provide precise histological findings. On the contrary, the use of radiofrequency signals may be more reliable for plaque characterization. A study using Integrated Backscatter-IVUS, which is able to depict the tissue characteristics of plaques, showed a significant reduction of lipid core volume after statin therapy.19 The present study showed consistent findings with those of animal studies that demonstrated a reduction of macrophage content and an increase of fibrosis after lipid lowering therapy.20,21 However, IVUS-VH further has the ability to define TCFA plaques as well as plaque composition in vivo. In fact, we clearly demonstrated not only a reduction in plaque volume, but also a remarkable change in relative lipid volume and an increase in fibrosis in TCFA.
The REVERSAL and ASTEROID trials utilizing conventional IVUS showed that intensive lipid-lowering treatment achieved reduced progression or regression of coronary atherosclerosis in stable patients after 18 to 24 months.16,17 The recent ESTABLISH study also revealed that early lipid lowering therapy by atorvastatin significantly reduced plaque volume in ACS after only 6 months.11 Our results of volumetric analysis are in line with these studies in terms of reduction of plaque volume. However, there was a discordant result regarding plaque composition after statin therapy. Although analysis of the entire patient population in this study showed no change in percent necrotic core between baseline and follow-up, a significant reduction in necrotic core percentage was observed in TCFA, but not in non-TCFA. The detailed mechanism of how statin treatment is more effective in vulnerable plaque than stable plaque during the early period of ACS could not be identified. Interestingly, a recent investigation from the REVERSAL trial demonstrated that constrictive remodeling of the arterial wall, representative of plaque stabilization, lacked an independent relation to LDL-C, but was positively related to CRP levels.22 These results suggest that the reduction of plaque burden associated with LDL-C lowering alone does not ensure constrictive remodeling following statin therapy. Moreover, the degree of CRP level reduction appears to be more important for plaque stabilization than that of LDL-C level reduction, especially during the early period of ACS. Furthermore, in the PROVE-IT trial, CRP levels rapidly diverged between treatment groups, likely accounting for the particularly rapid divergence of major cardiovascular events.10 Our data also demonstrated a significant correlation between change in percent necrotic core and CRP level. Thus, our observation reemphasizes the role of inflammation in plaque stabilization by statin therapy, particularly during the high risk period after ACS. However, the exact mechanism of this early benefit according to plaque stability cannot be established solely on the basis of these aforementioned results. To do so, biochemical markers or higher-resolution imaging techniques that can better define the mechanism of early stabilization after statin therapy are needed.
Several limitations should be taken into consideration. First, only 54 patients from a single center were enrolled; however, in order to obtain the high-quality images that allowed us to accurately identify a significant difference in plaque composition change, bifurcation lesions, lesions with severe angulations, heavily calcified lesions and lesions with poor quality image were excluded from this study. Thus, we precluded generalization of the findings to all patients with ACS. Second, an analysis of entire coronary segments was not performed. Measurement of the 10-mm subsegment with the most severe disease is substantially less rigorous compared with that of the entire segment. In addition, image matching of the target lesion between baseline and follow-up could increase measurement variability. Third, we could not include a control group who received a placebo; however, it was deemed ethically unacceptable in the setting of ACS. We compensated for the lack of a control group by blinding the information on the IVUS-VH. Fourth, VH-TCFA is not yet a validated surrogate for plaque prone to thrombosis. A study regarding the natural history of TCFA derived from IVUS-VH study, therefore, is needed to address this problem. It is also unclear whether TCFA detected by IVUS-VH was a true vulnerable plaque. In fact, a recent study indicated that this modality alone is not sufficient for detecting TCFA.23 However, in an effort to reduce biased selection, we demonstrated little intra-observer or inter-observer disagreement in the diagnosis of VH-derived TCFA. Finally, a long-term follow-up study is needed to establish earlier statin responses in TCFA than in non-TCFA.
In conclusion, despite these limitations, this is the first report, to our knowledge, to evaluate in vivo the relationship between plaque stability and early statin response, and to examine the influence of a systemic inflammatory marker on plaque composition. In conclusion, the present study provides valuable insight into understanding plaque composition change according to plaque stability, especially during the early period of ACS.
Figures and Tables
Fig. 1
(A) Change in mean percent necrotic core in the most diseased 10-mm subsegment during 6-month follow-up. Significant reduction in percent necrotic core in TCFA is observed. (B) Change in mean absolute necrotic core volume in the most diseased 10-mm subsegment during 6-month follow-up. Significant reduction in absolute necrotic core volume in TCFA is observed. TCFA, thin-cap fibroatheroma; NS, non-specific.

Fig. 2
Change in percentages of the four plaque components in TCFA (n=14) during 6-month follow-up. TCFA, thin-cap fibroatheroma.

Fig. 3
Representative examples of IVUS-VH images at largest necrotic core site for each plaque type. Baseline (left panel) and follow-up (right panel) images are displayed side by side. (A) Baseline image in the TCFA type. (B) Reduction of necrotic core is clearly observed after 6 months. (C) Baseline image in the non-TCFA type. (D) Increase of necrotic core is clearly observed. IVUS-VH, Intravascular Ultrasound-Virtual Histology; TCFA, thin-cap fibroatheroma.
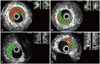
Fig. 4
Correlation between (A) change in necrotic core percentage and change in LDL-C level, as well as between (B) change in necrotic core percentage and change in CRP level. LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein.

Table 1
Baseline Clinical Characteristics
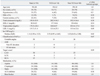
AMI, acute myocardial infarction; LAD, left anterior descending artery; RCA, right coronary artery; LCX, left circumflex artery; IQR, inter-quartile ranges; TCFA, thin-cap fibroatheroma; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; ACS, acute coronary syndrome.
Values are expressed as mean±SD.
ACKNOWLEDGEMENTS
This work was funded by Ulsan University Hospital (Biomedical Research Center Promotion Fund, UUH 2007-13). The authors would like to thank Jung Yong Park for his assistance and important contributions.
References
1. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000. 20:1262–1275.
2. Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993. 69:377–381.

3. Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002. 106:2200–2206.

4. Rodriguez-Granillo GA, García-García HM, Mc Fadden EP, Valgimigli M, Aoki J, de Feyter P, et al. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol. 2005. 46:2038–2042.

5. Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, Surmely JF, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006. 47:2405–2412.
6. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994. 344:1383–1389.
7. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002. 360:7–22.
8. FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet. 1999. 354:708–715.
9. Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001. 285:1711–1718.

10. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004. 350:1495–1504.

11. Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, et al. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation. 2004. 110:1061–1068.

12. Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O'Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000. 343:915–922.

13. Lee SG, Lee CW, Hong MK, Kim JJ, Park SW, Park SJ. Change of multiple complex coronary plaques in patients with acute myocardial infarction: a study with coronary angiography. Am Heart J. 2004. 147:281–286.

14. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005. 352:20–28.

15. Newby LK, Kristinsson A, Bhapkar MV, Aylward PE, Dimas AP, Klein WW, et al. Early statin initiation and outcomes in patients with acute coronary syndromes. JAMA. 2002. 287:3087–3095.

16. Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004. 291:1071–1080.

17. Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006. 295:1556–1565.

18. Schartl M, Bocksch W, Koschyk DH, Voelker W, Karsch KR, Kreuzer J, et al. Use of intravascular ultrasound to compare effects of different strategies of lipid-lowering therapy on plaque volume and composition in patients with coronary artery disease. Circulation. 2001. 104:387–392.

19. Kawasaki M, Sano K, Okubo M, Yokoyama H, Ito Y, Murata I, et al. Volumetric quantitative analysis of tissue characteristics of coronary plaques after statin therapy using three-dimensional integrated backscatter intravascular ultrasound. J Am Coll Cardiol. 2005. 45:1946–1953.

20. Armstrong ML, Megan MB. Arterial fibrous proteins in cynomolgus monkeys after atherogenic and regression diets. Circ Res. 1975. 36:256–261.

21. Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, et al. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation. 1998. 97:2433–2444.

22. Schoenhagen P, Tuzcu EM, Apperson-Hansen C, Wang C, Wolski K, Lin S, et al. Determinants of arterial wall remodeling during lipid-lowering therapy: serial intravascular ultrasound observations from the Reversal of Atherosclerosis with Aggressive Lipid Lowering Therapy (REVERSAL) trial. Circulation. 2006. 113:2826–2834.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download