Abstract
Purpose
Cerebral ischemic lesions are frequently observed after carotid artery stenting (CAS), and anti-platelet agents are used to prevent stent thrombosis and peri-procedural complications. However, despite the premedication, cerebral ischemic lesions are observed, suggesting that they may rather be related to anti-platelet resistance. We, therefore, investigated the effects of anti-platelet resistance on the development of cerebral ischemic lesions after CAS.
Materials and Methods
We retrospectively reviewed patients who received CAS and selected patients for whom brain MRI was performed within 24 hours after CAS and for whom anti-platelet resistance was checked. Anti-platelet resistance was examined by the VerifyNow system. We analyzed the correlation between anti-platelet resistance and cerebral ischemic lesions detected on follow-up MRI.
Results
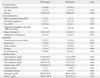
Among 76 patients, 45 (59.2%) developed new ischemic lesions after CAS. Twelve (15.8%) patients showed aspirin resistance and 50 (65.8%) patients showed clopidogrel resistance. Patients with a new ischemic lesion demonstrated a significantly greater frequency of clopidogrel resistance than those who had no new ischemic lesion (82.2% versus 41.9%, p=0.001). The frequency of aspirin resistance was not significantly different between the groups of patients with and without new ischemic lesions (20.0% versus 9.7%, p=0.340). In multivariate analysis, clopidogrel resistance was a significant risk factor for post-procedural cerebral ischemia.
Cerebral ischemic lesions are observed by MRI in roughly 50% of patients after carotid artery stenting (CAS).1,2 Generally, anti-platelet agents are used as premedication to prevent stent thrombosis and peri-procedural complications.3 Despite premedication with aspirin and clopidogrel, however, cerebral ischemic lesions are still frequently observed in patients upon brain diffusion-weighted MRI.1,2,4-6 Although the clinical implication of these cerebral ischemic lesions after CAS is not clear, they could potentially result in focal neurologic signs or cognitive dysfunction.1,2,4
Platelet function inhibition by aspirin or clopidogrel differs from individual to individual, and some patients suffer recurrent cerebrovascular or cardiovascular events, regardless of proper anti-platelet medication. In these cases, the patients may be clinically classified with anti-platelet resistance. A significant proportion of patients with coronary artery occlusive disease show aspirin or clopidogrel resistance, which is related with major adverse coronary events after percutaneous coronary intervention and recurrent atherothrombotic events in patients with acute myocardial infarction.7-9 In contrast to coronary artery occlusive disease, the role of anti-platelet resistance in carotid artery disease has not been well characterized. The reported prevalence of aspirin and clopidogrel resistance in cerebrovascular intervention ranges from 2 to 21% for aspirin and 43-52% for clopidogrel.10-12 However, these studies included only a few CAS patients (6.6%,10 16%,11 33.7%12) and did not provide any information about the clinical significance of anti-platelet resistance in cerebrovascular stent placement. Therefore, we performed this study to determine the clinical significance of anti-platelet resistance in patients who underwent CAS, by investigating if there was any correlation between anti-platelet resistance and new cerebral ischemic lesions detected by 3.0T brain MRI after CAS.
We retrospectively enrolled 76 patients who satisfied the following criteria from January 2007 to May 2011 in our registry: premedication of dual anti-platelet agents (aspirin and clopidogrel) at least 7 days before CAS, pre-stent brain MRI within 60 days, post-stent brain MRI within 24 hours, and aspirin and clopidogrel resistance test before CAS or within 2 days after CAS. Cases of emergent carotid stent insertion, those without pre or post-stent brain MRI, and those without anti-platelet resistance tests for both aspirin and clopidogrel were excluded. We obtained the past history, clinical features, and laboratory findings of patients by review of their medical records. We excluded the patients who took proton pump inhibitors. This study was approved by the Severance Hospital Institutional Review Board of the Yonsei University Health System in Seoul, Korea.
CAS was performed in patients with symptomatic (ischemic stroke or transient ischemic attack related to relevant artery) internal carotid artery (ICA) stenosis of 50% or more or asymptomatic stenosis of 70% or more according to the North American Symptomatic Carotid Endarterectomy Trial criteria on digital subtraction cerebral angiography. Four experienced neurointerventionist (S.H. Suh, D.J. Kim and B.M. Kim in radiology, P.K. Min in cardiology) managed all the procedures. We introduced a 90-cm-long 7 F or 8 F guiding catheter into the femoral sheath with a 120-cm-long 4 F or 5 F diagnostic catheter coaxially. Then, we removed the diagnostic catheter after appropriate positioning of the guiding catheter proximal to the stenotic lesion.13 We did not perform aortogram separately. Pre-stenting balloon angioplasty was performed in patients with severe stenosis. In cases of difficult penetration of the cerebral protection device (CPD), balloon angioplasty was initially performed using a 1.5-2 mm balloon catheter before placement of the CPD. After placing the CPD, a self-expandable stent [Protege (ev3, Irvine, CA, USA), Precise (Cordis endovascular, Miami Lakes, FL, USA), or Wallstent (Boston Scientific, Natick, MA, USA)] was deployed in the proximal ICA or the distal common carotid artery, and post-stenting angioplasty was done optionally when residual stenosis (more than 50%) was noted on angiography. The patients received a bolus injection of 3000-4000 IU heparin just before the start of the therapeutic procedure. The time intervals from pre-stent MRI to diagnostic cerebral angiography, pre-stent MRI to CAS, pre-stent MRI to post-stent MRI and diagnostic cerebral angiography to post-stent MRI were determined for patients who underwent CAS. If one-stage CAS was performed, the time interval from the first diagnostic angiography to CAS was considered as zero. At least 7 days before the procedure, all patients received 75 mg of clopidogrel and 100 mg of aspirin daily. Neurologic evaluation was performed before and at 24 hours after the procedure.
All MRI examinations were performed on a 3.0T MRI system (Signa 3.0T, General Electric, Milwaukee, WI, USA; Achieva 3.0T, Philips Medical Systems, Best, the Netherlands). Diffusion-weighted images (DWI) were obtained using the following parameters: TR/TE 9000/81.4 ms, matrix 128×128, FOV 240×240 mm, slice thickness 4 mm, b=1000 s/mm2. The resulting voxel volumes were 14 mm3. Acute probable ischemic lesions were defined as a hyperintense lesion on DWI or corresponding hypointense lesion in apparent diffusion coefficient maps. A pre-stent MRI was performed within 60 days before the procedure. Post-stent brain DWI was carried out routinely within 24 hours after stent placement regardless of the presence of neurologic symptoms after CAS. When newly developed lesions were found after the procedure by comparing pre- and post-brain DWI, the case was defined as DWI-positive. If there were no newly developed lesions on follow-up DWI, the case was defined as DWI-negative. The number, size and location of the DWI-positive lesions according to the vascular territory and CAS side were recorded.14
All patients received 75 mg of clopidogrel and 100 mg of aspirin for at least 7 days before undergoing aspirin and clopidogrel resistance tests. Aspirin resistance and clopidogrel resistance were determined by the VerifyNow system (Accumetrics, San Diego, CA, USA). The VerifyNow assay is a whole blood, light transmission-based optical detection assay that measures platelet aggregation in a cartridge containing fibrinogen-coated beads. An aspirin reaction unit (ARU) value ≥550 was defined as aspirin resistance.15 Clopidogrel resistance was expressed in P2Y12 reaction units (PRU). A PRU value ≥240 was defined as clopidogrel resistance.16
Statistical analyses were performed using PASW Statistics 18 (IBM, Chicago, IL, USA). Continuous variables are presented as means with standard deviations and categorical variables are presented as frequencies and percentages. Variables were compared by independent t-test, chi-square test, Mann-Whitney U test and Fisher's exact test. Univariate analysis was performed and factors with a p-value less of than 0.1 in univariate analysis were included in multivariate logistic regression analysis. A two-tailed value of p<0.05 was considered statistically significant.
Seventy-six patients were selected for this study. The mean age of the patients was 68.11±7.72 years, and 56 (73.7%) patients were males. In total, 39 patients (51.3%) underwent one-stage CAS without a previous diagnostic angiography procedure. Fifty seven (75.0%) patients had symptomatic carotid artery stenosis, 21 (36.8%) patients had a history of stroke confirmed by brain CT or MRI and 36 (63.2%) patients had a transient history of ischemic attack related to the carotid stent side within 6 months before CAS. Pre-stent balloon angioplasty was performed in 61 (80.3%) patients, before placement of the CPD in 7 patients and after placement of the CPD in 54 patients. Seventy patients (92.1%) used the CPD. Post-stent balloon angioplasty was performed in 47 (61.8%) patients. The mean ARU and PRU values of all examined patients were 466.32±79.33 and 257.28±81.84, respectively. Twelve (15.8%) patients were resistant to aspirin, while 50 (65.8%) patients were resistant to clopidogrel, and 9 (11.8%) patients were resistant to both aspirin and clopidogrel. The frequency of aspirin resistance was similar to that of resistance to both drugs.
Among 76 patients, 45 (59.2%) patients had DWI-positive lesions; 40 patients were classified with asymptomatic stroke and 5 patients with symptomatic stroke. Eleven (24.4%) patients had a single DWI-positive lesion, 16 (35.6%) patients had 2 to 5 lesions, and 18 (40.0%) patients had more than 5 lesions. In 17 (37.8%) patients, lesions were located only on the ipsilateral side to stent placement. In contrast, lesions in 14 (31.1%) patients were detected in both the ipsilateral and non-ipsilateral vascular territory, and lesions in 14 (31.1%) patients were detected only in the non-ipsilateral vascular territory relative to the CAS. The DWI-positive lesions were frequently detected in the cerebral cortex and middle cerebral artery (MCA) territory. The DWI-positive lesion size was less than 5 mm in 164 (72.8%), 5 mm to 10 mm in 35 (15.6%) and larger than 10 mm in 26 (11.6%).
All 5 (6.6%) symptomatic stroke cases after the procedure were from the symptomatic carotid artery stenosis group and had minor neurological deficits (National Institutes of Health Stroke Scale score <4). These 5 patients were all DWI-positive and demonstrated aspirin and clopidogrel resistance. The location of the lesions according to CAS side was the ipsilateral MCA in 3 patients, the bilateral MCA in one patient, and the ipsilateral MCA and posterior inferior cerebellar artery in one patient.
In patients with symptomatic carotid stenosis, the time interval between ischemic symptom onset and CAS was 19.69 days, and there was no time difference between DWI-positive patients (22.67±20.04 days) and DWI-negative patients (13.89±14.07 days) (p=0.063). For all patients, the duration of aspirin and clopidogrel medication before CAS was a mean of 11.57 days, and there was also no time difference between DWI-positive patients (11.86±4.64 days) and DWI-negative patients (11.16±2.33 days) (p=0.439). In Spearman correlation analysis, there was no correlation between degrees of carotid stenosis and ARU (p=0.985, rho=-0.002) or PRU (p=0.189, rho=0.152) values.
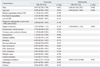
There was no difference in frequency of aspirin resistance between patients with and without DWI-positive lesions (p=0.340); however, clopidogrel resistance was detected more frequently in patients with DWI-positive lesions than patients without (82.2% versus 41.9%, p=0.001) (Table 1). After adjusting for age, gender, and degree of stenosis, clopidogrel resistance was a significant predictor of DWI-positive lesions after CAS upon multivariate analysis (odds ratio: 6.804; 95% confidence interval: 2.225-20.806; p=0.001) (Table 2).
Periprocedural microembolization is an important risk for patients treated by angioplasty and stent placement of high-grade carotid stenosis. Atheromatous plaque and superimposed thrombi are the main source of microemboli during CAS.17 Therefore, anti-platelet premedication and maintenance is very important for preventing peri-procedural ischemic events. Recent investigations suggested that anti-platelet resistance is associated with cardiovascular or cerebrovascular events.9,18,19 Patients with aspirin resistance have been shown to have increased mortality and be at greater risk for acute coronary syndrome, vascular intervention failure, or new cerebrovascular events than aspirin-sensitive patients.18 Furthermore, clopidogrel resistant patients tend to have more recurrent cardiovascular events and a higher rate of subacute stent thrombosis after coronary stenting, compared with clopidogrel sensitive patients.9,19
We investigated the relationship between anti-platelet resistance and the presence of new cerebral ischemic lesions that developed after CAS. In our study, 15.8% of patients were aspirin resistant and 65.8% were clopidogrel resistant, which was comparable to previous studies.10-12 Furthermore, the frequency of aspirin resistance was similar to that of resistance to both aspirin and clopidogrel in our study, consistent with a previous report.20 In aspirin resistance patients, platelets appear to have increased sensitivity to ADP-induced glycoprotein IIb/IIIa activation. These hyper-reactive platelets may be less sensitive to inhibition by clopidogrel.21,22
In our study, the frequency of clopidogrel resistance was significantly higher in DWI-positive patients than in DWI-negative patients. Therefore, medications which have pharmacological mechanisms different from that of clopidogrel should be considered, if clopidogrel resistance is detected in patients with CAS.
In this study, cerebral ischemic lesions were detected in 59.2% of patients after CAS, while a CPD was used in 92.1% of the patients. When performing CAS, cerebral ischemic lesions are frequently detected on DWI, which is considered a very effective tool for detecting acute ischemic brain lesions.1,2,4-6,14,23-25 Despite the use of a CPD to reduce embolic complications during CAS, post-procedural DWI-positive lesions were detected in 15 to 40% of cases in previous studies.2,26-29 Firstly, one possible reason for the high rate of new ischemic lesions detected after CAS is that new lesions may have developed before the stent placement. That is, we cannot exclude the possibility that new ischemic lesions developed spontaneously after the index stroke or by the time of the first diagnostic cerebral angiographic procedure. However, in our study, about half of the patients underwent one stage CAS and there was no difference in the time interval from pre-stent MRI to diagnostic cerebral angiography, pre-stent MRI to CAS, pre-stent MRI to post-stent MRI and diagnostic cerebral angiography to post-stent MRI between the DWI-positive and negative groups. Therefore, the possibility of ischemic lesions not related to the CAS itself might not be that high.
Another possible reason for the high rate of new ischemic lesions after CAS in our study may be the use of 3.0T MRI; previous studies used 1.5T MRI.1,2,14,27-29 Most important difference between 1.5T MRI and 3.0T MRI is the performance of the gradient subsystem for controlling the quality of the DWI. The improved gradient subsystem in 3.0T MRI allows the voxel size and echo time of the DWI sequence to be decreased, thereby increasing resolution. This could make 3.0T MRI more accurate for pathologic lesion detection than 1.5T MRI, especially when performing DWI.30
In our study, minor ischemic strokes occurred in five patients within 24 hours after CAS. This result was comparable to previous studies, which reported 4.5-7.2% symptomatic neurological complication after CAS.2,5,6,23 The fact that all of these patients in our study showed resistance to pregiven anti-platelet agents emphasizes the clinical significance of anti-platelet resistance in CAS.
Our study had several limitations. There was a possibility of selection bias, because patients who did not undergo post-stent MRI or ant-platelet resistance test were excluded. However, we routinely recommended both studies to all patients with CAS, regardless of patient symptoms or signs after CAS. In addition, we did not examine the long-term clinical significance of anti-platelet resistance after CAS. These limitations are due to retrospective design of the study.
In conclusion, the evaluation of anti-platelet resistance, especially clopidogrel resistance, may be useful to predict the development of cerebral ischemia after CAS. We, therefore, recommend routine check-up of anti-platelet resistance. Long-term prospective studies using clinical outcome measurements are needed to clarify the significance of anti-platelet resistance in CAS.
ACKNOWLEDGEMENTS
This work was supported by a Faculty Research Grant from Yonsei University College of Medicine (6-2010-0120).
References
1. Poppert H, Wolf O, Resch M, Theiss W, Schmidt-Thieme T, Graefin von Einsiedel H, et al. Differences in number, size and location of intracranial microembolic lesions after surgical versus endovascular treatment without protection device of carotid artery stenosis. J Neurol. 2004. 251:1198–1203.

2. Hammer FD, Lacroix V, Duprez T, Grandin C, Verhelst R, Peeters A, et al. Cerebral microembolization after protected carotid artery stenting in surgical high-risk patients: results of a 2-year prospective study. J Vasc Surg. 2005. 42:847–853.

3. Olsen TS, Langhorne P, Diener HC, Hennerici M, et al. European Stroke Initiative Executive Committee. EUSI Writing Committee. European Stroke Initiative Recommendations for Stroke Management-update 2003. Cerebrovasc Dis. 2003. 16:311–337.

4. Gossetti B, Gattuso R, Irace L, Faccenna F, Venosi S, Bozzao L, et al. Embolism to the brain during carotid stenting and surgery. Acta Chir Belg. 2007. 107:151–154.

5. Schlüter M, Tübler T, Steffens JC, Mathey DG, Schofer J. Focal ischemia of the brain after neuroprotected carotid artery stenting. J Am Coll Cardiol. 2003. 42:1007–1013.

6. Hauth EA, Jansen C, Drescher R, Schwartz M, Forsting M, Jaeger HJ, et al. MR and clinical follow-up of diffusion-weighted cerebral lesions after carotid artery stenting. AJNR Am J Neuroradiol. 2005. 26:2336–2341.
7. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Barrera-Ramirez C, Sabaté M, et al. Identification of low responders to a 300-mg clopidogrel loading dose in patients undergoing coronary stenting. Thromb Res. 2005. 115:101–108.

8. Marcucci R, Paniccia R, Antonucci E, Gori AM, Fedi S, Giglioli C, et al. Usefulness of aspirin resistance after percutaneous coronary intervention for acute myocardial infarction in predicting one-year major adverse coronary events. Am J Cardiol. 2006. 98:1156–1159.

9. Matetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Goldenberg I, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004. 109:3171–3175.
10. Prabhakaran S, Wells KR, Lee VH, Flaherty CA, Lopes DK. Prevalence and risk factors for aspirin and clopidogrel resistance in cerebrovascular stenting. AJNR Am J Neuroradiol. 2008. 29:281–285.

11. Reavey-Cantwell JF, Fox WC, Reichwage BD, Fautheree GL, Velat GJ, Whiting JH, et al. Factors associated with aspirin resistance in patients premedicated with aspirin and clopidogrel for endovascular neurosurgery. Neurosurgery. 2009. 64:890–895.

12. Lee DH, Arat A, Morsi H, Shaltoni H, Harris JR, Mawad ME. Dual antiplatelet therapy monitoring for neurointerventional procedures using a point-of-care platelet function test: a single-center experience. AJNR Am J Neuroradiol. 2008. 29:1389–1394.

13. Montorsi P, Galli S, Ravagnani P, Ruchin P, Lualdi A, Fabbiocchi F, et al. Randomized trial of predilation versus direct stenting for treatment of carotid artery stenosis. Int J Cardiol. 2010. 138:233–238.
14. Jaeger HJ, Mathias KD, Hauth E, Drescher R, Gissler HM, Hennigs S, et al. Cerebral ischemia detected with diffusion-weighted MR imaging after stent implantation in the carotid artery. AJNR Am J Neuroradiol. 2002. 23:200–207.
15. Patti G, Nusca A, Mangiacapra F, Gatto L, D'Ambrosio A, Di Sciascio G. Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol. 2008. 52:1128–1133.
16. Mangiacapra F, Patti G, Peace A, Gatto L, Vizzi V, Ricottini E, et al. Comparison of platelet reactivity and periprocedural outcomes in patients with versus without diabetes mellitus and treated with clopidogrel and percutaneous coronary intervention. Am J Cardiol. 2010. 106:619–623.

17. Piñero P, González A, Martínez E, Mayol A, Rafel E, González-Marcos JR, et al. Volume and composition of emboli in neuroprotected stenting of the carotid artery. AJNR Am J Neuroradiol. 2009. 30:473–478.

18. Krasopoulos G, Brister SJ, Beattie WS, Buchanan MR. Aspirin "resistance" and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008. 336:195–198.

19. Müller I, Besta F, Schulz C, Massberg S, Schönig A, Gawaz M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost. 2003. 89:783–787.

20. Oqueli E, Hiscock M, Dick R. Clopidogrel resistance. Heart Lung Circ. 2007. 16:Suppl 3. S17–S28.

21. Wang TH, Bhatt DL, Topol EJ. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur Heart J. 2006. 27:647–654.

22. Lev EI, Patel RT, Maresh KJ, Guthikonda S, Granada J, DeLao T, et al. Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J Am Coll Cardiol. 2006. 47:27–33.

23. McDonnell CO, Fearn SJ, Baker SR, Goodman MA, Price D, Lawrence-Brown MM. Value of diffusion-weighted MRI during carotid angioplasty and stenting. Eur J Vasc Endovasc Surg. 2006. 32:46–50.

24. Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol. 1995. 37:231–241.

25. Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, et al. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke. 2002. 33:2206–2210.

26. du Mesnil de Rochemont R, Schneider S, Yan B, Lehr A, Sitzer M, Berkefeld J. Diffusion-weighted MR imaging lesions after filter-protected stenting of high-grade symptomatic carotid artery stenoses. AJNR Am J Neuroradiol. 2006. 27:1321–1325.
27. Jaeger HJ, Mathias KD, Drescher R, Hauth E, Bockisch G, Demirel E, et al. Diffusion-weighted MR imaging after angioplasty or angioplasty plus stenting of arteries supplying the brain. AJNR Am J Neuroradiol. 2001. 22:1251–1259.
28. Piñero P, González A, Mayol A, Martínez E, González-Marcos JR, Boza F, et al. Silent ischemia after neuroprotected percutaneous carotid stenting: a diffusion-weighted MRI study. AJNR Am J Neuroradiol. 2006. 27:1338–1345.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download