Abstract
The desired effect of vaccination is to elicit protective immune responses against infection with pathogenic agents. An inactivated influenza vaccine is able to induce the neutralizing antibodies directed primarily against two surface antigens, hemagglutinin and neuraminidase. These two antigens undergo frequent antigenic drift and hence necessitate the annual update of a new vaccine strain. Besides the antigenic drift, the unpredictable emergence of the pandemic influenza strain, as seen in the 2009 pandemic H1N1, underscores the development of a new influenza vaccine that elicits broadly protective immunity against the diverse influenza strains. Cold-adapted live attenuated influenza vaccines (CAIVs) are advocated as a more appropriate strategy for cross-protection than inactivated vaccines and extensive studies have been conducted to address the issues in animal models. Here, we briefly describe experimental and clinical evidence for cross-protection by the CAIVs against antigenically distant strains and discuss possible explanations for cross-protective immune responses afforded by CAIVs. Potential barriers to the achievement of a universal influenza vaccine are also discussed, which will provide useful guidelines for future research on designing an ideal influenza vaccine with broad protection without causing pathogenic effects such as autoimmunity or attrition of protective immunity against homologous infection.
Influenza viruses continue to change their antigenicity by successfully evading the host immunity acquired by previous vaccinations or natural exposures to infections and claim the lives of 250,000 to 500,000 people worldwide annually. The antigenic drift by genetic mutations in the influenza viral genome not only leads to the emergence of antiviral drug-resistant strains,1,2 but also evades antibody-mediated viral neutralization (VN).3 Vaccination remains the most cost-effective countermeasure against influenza virus infection. The effect of vaccination relies on the induction of high levels of neutralizing antibodies specific to the viral surface proteins, hemagglutinin (HA) and neuraminidase (NA), which mediates the initial attachment of the virus to specific cellular receptors on the cell surface promoting the entry of the virus into the target cell and the cleavage of the sialic acid moiety from the receptor permitting the release of progeny virus particles, respectively. Influenza vaccines are prepared each year with the aim of matching the strains that would circulate in that season. The current inactivated influenza vaccines contain three distinctive antigens from two influenza A virus strains (H1N1 and H3N2) and one B strain as recommended by the World Health Organization (WHO). While providing protective immune responses against homologous and closely related virus strains, they rarely protect against antibody-escape variants of seasonal influenza viruses or newly circulating strains in subsequent influenza seasons, hence necessitating an annual update of new HA and NA antigens. Furthermore, as seen in the previous outbreaks of the highly pathogenic avian H5N1 influenza virus (HPAI), a completely new strain against which contemporary vaccines provide little or no protection could emerge from non-human avian reservoirs.4-6 Although a sustained human-to-human transmission of the H5N1 HPAI has not yet been observed, it should be noted that three major influenza pandemics of the 20th century were all caused by influenza A viruses originating from birds, calling for constant vigilance and monitoring. Recent demonstration that guided mutations in the HA of the HPAI markedly increased the transmission efficiency in mammalian hosts partially mirrors similar antigenic drifts in nature as well.7-9 Meanwhile, the sudden emergence and rapid transmission of the 2009 pandemic H1N1 influenza virus (pdmH1N1) was recorded as the first pandemic in the 21st century. The pdmH1N1 itself was shown to have a unique genetic constellation that has not been previously reported.10,11 This raised a possibility that further genetic reassortment of the virus with other virulent strains (antigenic shift) could generate other novel pandemic strains with a high virulence and human transmissibility. In support of this hypothesis, experimental evidence showed a considerable genetic compatibility between the HPAI and the pdmH1N1, where the reassortants between the two viruses led to high transmission ability among mammalian hosts.12,13
The persistent threats posed by antigenically diverse and rapidly evolving influenza viruses heighten the interests in the development of cross-protective influenza vaccines and ultimately a universal vaccine that could provide protection against antigenic drift and shift strains of influenza. Relevant to this issue, the cross-reactive cytotoxic T lymphocytes (CTLs) and broadly neutralizing antibodies directed to the conserved domains of influenza viral proteins have been proposed as the most likely triggers to eliciting cross-protective immunity across different influenza strains. T cell-mediated cross-reactivity is frequently observed in influenza infections among different strains of the same virus and, in rare occasions, even among unrelated viruses such as hepatitis C virus and Epstein-Barr virus.14 Numerous reports on influenza viruses have demonstrated that the T cell responses are crucial for viral clearance by removing the virus-infected cells from the host.15-18 However, the CTL responses are considered a 'double-edged sword' since, while providing cross-protective immune responses, they sometimes aggravate disease symptoms by having a pathogenic effect.19-21 However, broadly neutralizing antibodies have recently emerged as reliable effectors for the cross-reactivity against the influenza viruses; also providing a promising avenue to the development of cross-protective influenza vaccines. The two major principles underlying cross-reactivity provide the cornerstone for developing cross-protective influenza vaccines.
In light of cross-protection, cold-adapted live attenuated influenza vaccines (CAIVs) are of much interests since they can mount all phases of immune responses, including systemic and local humoral responses as well as cell-mediated immunity. Along with the CTLs and the broadly reactive antibodies, the intranasal administration of the CAIVs stimulates the induction of secretory IgA antibodies in the respiratory tract, which crucially contributes to cross-protection.
Here, we review the experimental and clinical evidence for cross-reactive immunity afforded by the CAIVs, and further discuss possible mechanisms underlying the cross-protection by the vaccines. In addition, important considerations for the development of more effective and cross-protective influenza vaccines are described; therefore, tipping the balance of the CTL responses from a pathogenic to a beneficial cross-protective immune response.
Inactivated influenza vaccines have been most widely used in preventing influenza infection in humans for more than fifty years. There are two types of inactivated vaccines currently used clinically. Split-virus vaccines are prepared by disrupting the whole virions containing all viral particles and the ssRNA genome, whereas subunit vaccines contain the highly purified HA and NA proteins-the two most immunogenic surface glycoproteins. Inactivated influenza vaccines preferentially induce serum IgG antibodies directed to HA, which neutralize the virus by binding the globular head domain of the HA and preventing the initial attachment of the virus to cellular receptors on the surface membrane.
In general, inactivated vaccines are poorly immunogenic, requiring at least two separate vaccinations and appropriate adjuvants to induce sufficiently protective immune responses.22,23 Since protection by the vaccines highly depends on the induction of the neutralizing antibodies against the HA, the vaccines need to be reformulated yearly according to the antigenic changes in a subsequent season. Occasionally, the vaccine strains fail to properly match the circulating strains; therefore, the vaccine effectiveness is significantly reduced, which was documented most apparently during 1997-1998.24,25 Seasonal influenza vaccines could be prepared in advance upon recommendation from the WHO or CDC based on global surveillance. Pandemic outbreaks, however, often accompany an antigenic shift resulting from genetic reassortment between more than two different strains, as seen in the pdmH1N1,10,11 rendering the previous vaccinations completely ineffective against the new pandemic strains.
The diversity of reservoirs, frequent animal-to-human transmission, and highly variable nature of the influenza virus led us to develop alternative vaccination strategies to enhance the broadly reactive antibodies and the CTL responses. For instance, immunization with a novel immunogen comprising the conserved stalk domain of the HA and lacking the variable head domain provided a broad spectrum of protection against diverse virus strains in mice.26 In parallel, alternative vaccine strategies were tested for their potential of improving the CTL responses. Since the CTL responses are directed to antigenic peptides processed in the cytoplasm of the antigen presenting cells, endogenous protein synthesis is essential to inducing functional CTL responses against the influenza viral infection. Immunization with a DNA construct encoding influenza viral proteins leads to in situ synthesis and processing of the viral antigens in the cytoplasm of the injected cells, which are then loaded into the major histocompatiblity complex (MHC) class-I molecules to subsequently stimulate the CTL responses. DNA immunization against influenza antigens, including HA, NP, M1, and M2, has shown protective immunity against homologous and heterologous infections in various animal models,27-29 but safety concerns remain with regard to the use of DNA for mass vaccination purposes.30
With respect to cross-protective immune responses, annual use of inactivated vaccine may hamper the development of the influenza virus-specific cross-reactive CTLs.31-34 In mice and ferrets, the use of inactivated A/H3N2 vaccines prevented the induction of heterosubtypic immunity to a lethal infection with influenza A/Indonesia/5/2005 (H5N1), which was found to correlate with reduced CTL responses.32-34 Similar patterns were also observed in humans, in which children who had received annual influenza inactivated vaccines did not show the age-dependent increase in the frequency of influenza-specific CTLs.31 It was assumed in those reports that the annual vaccinations early in life, particularly with inactivated vaccines, might lead to skewed immune responses preferentially enhancing the humoral responses. Therefore, in those reports, the use of the live attenuated influenza vaccine - able of inducing the virus-specific CTLs as well as the humoral responses - was recommended to the young children, in light of inducing cross-protective immunity to render them protected against antigenic variants or pandemic threats as well.
As an attractive alternative to the inactivated vaccines, the CAIVs have been used in humans against annual seasonal influenza virus infections since 2003. In addition, the CAIVs against a pandemic or a potential pandemic strain, such as the pdmH1N1 and H5N1 HPAI, were developed and evaluated for their safety and efficacy in animal models.35-40 Besides inherent issues of safety and efficacy, the CAIV provides specific advantages pertaining to cross-protection. The CAIVs are delivered via intranasal administration mimicking a natural infection by the influenza virus and hence is able to mount the local antiviral immunity, which has been thought to provide cross-protection. Such cross-protective immunity could also be effectively elicited by intranasal delivery even with the inactivated vaccines.41,42 Furthermore, in addition to humoral antibody responses directed to surface antigen proteins, the CAIVs replicate to a limited extent in the respiratory tracts and deliver internal viral components, which are then subjected to antigen presentation pathways involving MHC class-I molecules, consequently stimulating the CTL responses. Taken together, the CAIVs appear to encompass almost all immune responses that would not be expected from inactivated or DNA vaccines. However, the CAIVs may be less competent than inactivated or DNA vaccine in eliciting one particular phase of an immune response. For example, subunit vaccines are composed of highly purified surface antigens; therefore, are able of inducing higher serum IgG antibody titers than the CAIVs. It was reported that the CAIVs induced higher levels of local IgA antibody but lower levels of serum hemagglutinin inhibition (HI) antibody than inactivated vaccines, which became more prominent in the elderly people aged 50 and more, suggesting the use of both vaccines in combination for achieving optimal protection efficacy in the group.43,44 The DNA vaccine encoding internal viral proteins might be more specialized for stimulating the CTLs than the CAIVs, due to the over expression of the selected proteins under the control of a strong eukaryotic promoter such as the cytomegalovirus promoter. However, each of these strategies cannot stimulate the same wide range of immune responses expected from the CAIVs. Among several advantages presented by the CAIVs, stimulation of broad immunity against heterologous strains is of particular interest, especially because it may open exciting opportunity of developing a 'universal vaccine' that confers a wide range of protection against diverse antigenic strains, which remains an ultimate goal in the vaccine research field.
It has been well established that natural infection with an influenza virus results in protective immunity against reinfection with the same virus strain (strain-specific immunity), drift viruses within the same subtype (subtype-specific immunity) and even with different subtype viruses (heterosubtypic immunity), albeit with less strength than against a homologous strain.45 Such commonly observed cross-protection by natural infection serves as a working model of protection afforded by the CAIVs. The CAIVs have been extensively investigated for their genetic stability, immunogenicity, and protective efficacy in a number of preclinical and clinical trials, before becoming licensed for human use as a trivalent formulation containing two A types (H1N1 and H3N2) and one B type strain. To date, a number of reports have shown that immunization with the CAIVs elicits varying degrees of cross-protective immunity to heterologous influenza virus strains (Table 1). Those reports were initially focused mainly on the subtype-specific immunity that was demonstrated within the same subtype, between the vaccine and its drifted variants. In field trials for humans, vaccinations with the CAIVs induced serum HI antibodies against a drifted strain that was not contained in the vaccine, and contributed to a significant reduction of illness by infection with the drift virus, as compared to the placebo recipients.46-49 Subsequently, it was reported that in humans the degree of cross-protection by the CAIVs could vary depending on the age of recipients, with higher efficacy reported in children than adults.50,51 Realizing that the cross-protective immunity depends on the CTLs specific to the conserved epitopes of influenza viral proteins, efforts to illuminate the breadth of the cross-reactivity afforded by the CAIVs were extended to heterosubtypic immunity operating among different subtypes. In mice, cold-adapted H3N2 vaccine provided partial resistance to H1N1 infection through the CTL responses, the depletion of the CTLs cancelling out the protection against the challenge, highlighting the importance of the CTL responses to eliciting cross-protection.52
With the HPAI emerging as serious pandemic threats to human health, several types of contemporary seasonal vaccines were assessed for their cross-reactivity against the HPAI,53-55 and yet there has been no published data addressing the cross-reactivity between the seasonal CAIVs and the HPAI. Heterosubtypic cross-protection against the HPAI conferred by cold-adapted 2009 pdmH1N1 vaccines40,56 indirectly suggests the possible cross-reactivity between them. It is not surprising that immunization with the A/Ann Arbor/6/60 ca (H3N2), the CAIV donor strain itself, provided partial protection against the HPAI infection in mice.35 This result implies again that immunization with the cold-adapted donor strain can provide a certain degree of partial protection against heterologous infections, regardless of the subtype of challenging virus. This basal level of cross-reactivity afforded by the CAIV donor strains should present a practical means to develop the universal vaccine. Relevant to the issue of cross-reactive immune responses, enormous antigenic diversity in the HPAI makes it imperative that a H5N1 vaccine should cover a broad range of H5N1 variants from different clades or subclades.57,58 This cross-clade protection was examined in various animal models including mice, ferrets, and nonhuman primates, in which one or two doses of immunizations with the H5N1 CAIV elicited protective antibody responses against both homologous and heterologous strains with different clade or subclade HPAIs.35,36,59
The sudden emergence and global circulation of the pdmH1N1 with an unprecedented speed was against common prediction that H5N1 HPAI would be the next pandemic strain. Fortunately, pdmH1N1 caused fewer deaths than previous pandemics before moving into its postpandemic period. In parallel with global efforts to develop CAIVs against the pdmH1N1,39,40,60,61 many researchers also evaluated the cross-reactivity between the seasonal CAIVs and the pdmH1N1, with the hope that the seasonal CAIVs that contained H1N1 subtype would elicit protection against the pdmH1N1. The comparative studies with seasonal CAIVs and inactivated vaccines revealed the superior protection against pdmH1N1 by the seasonal CAIVs, and the cross-reactive CTL responses were proposed to be the most likely correlates for this protection.38,62,63 Of note, in humans with prior exposure to the pdmH1N1 or immunized with inactivated H1N1 vaccine, broadly cross-reactive antibodies dominated the human B cell responses against heterologous influenza strains,64,65 suggesting that the HA of the pdmH1N1 might carry many conserved epitopes and was able to preferentially elicit antibodies specific to those epitopes. Such broad reactivity of the HA of the pdmH1N1 was further confirmed in an animal model study, in which immunization with the pdmH1N1 CAIVs conferred high level of cross-protection against the seasonal and the HPAI infections in mice.40,56
In this section, we discuss currently proposed mechanisms for the cross-reactivity of the CAIVs (Table 2) suggested in the references discussed above, which could be further extended to the cross-reactivity seen in other live attenuated vaccine strategies;66 however, with a varying degree of contribution of each factor depending on the attenuation tools employed.
The CTLs are MHC class-I-restricted CD8 T cells that play a central role in killing the host cells infected with viruses or intracellular microbial pathogens.67 Since the CTLs recognize foreign peptides that are degraded in the cytoplasm and loaded into the MHC class I molecules, influenza internal components constitute the primary resources of antigenic peptides pool for the influenza specific CTLs. However, the two surface glycoproteins (HA and NA) are expressed by the ribosomes on the rough endoreticulum reticular and remain embedded in the lipid membrane until translocated to the cytoplasmic membrane. These surface antigens are processed and loaded into the MHC class-II molecules in endocytic vesicle, and then presented to CD4 T cells for stimulation of antibody production by the B cell.67 It has been well recognized that non-glycoproteins are highly conserved according to the type (A, B, or C), and therefore the CTLs specific to a certain influenza strain will elicit a broad spectrum of responsiveness to diverse viral subtypes within the same type. Indeed, several reports showed that the influenza virus-specific CTLs were directed against the NP and M1 protein,18,68,69 based on which the influenza viruses are divided into three types (A, B, and C). Moreover, many studies suggested that the cross-reactive immunity between different subtypes were mediated by the CTLs, as deduced from diverse combinations of priming and challenge experiments in animal models.45 It is now established that the cross-reactive CTLs play crucial roles for viral clearance and the decrease the morbidity associated with infection, although it alone cannot prevent infection. Several studies aimed to identify potently immunogenic and conserved T cell epitopes embedded in influenza viral proteins in addition to NP and M1 and aimed to design more broadly protective vaccines.70-72 As demonstrated in the CAIVs, the CTL responses were demonstrated to be critical factors responsible for eliciting cross-protection against heterosubtypic infections. The pdmH1N1 CAIV protected the immunized mice from heterologous infections with the seasonal H1N1 and the avian H5N1 virus, even without measurable HI or viral neutralization (VN) antibody titer, inducing significant specific CTL responses to the viruses, which suggested the important role of the CTL responses for protection.56 Although the reports summarized in Table 1 does not fully represent the data on the CTL responses, it is highly predictive that the CTL responses would participate in the cross-protection, which would contribute to more significantly to heterosubtypic protection than to subtype-specific or cross-clade protections.
Administrated via the nasal route, a typical entry site used by the influenza virus, the CAIVs are able to induce local antiviral immunity in the upper and lower respiratory tracts (RT). Of particular importance with respect to the cross-protection is the induction of secretory IgA antibodies (sIgA) in the RT. The sIgA is a major humoral mediator of mucosal immunity and, like IgM, has a tendency to form a polymeric structure that is essential for transport through the epithelium. Moreover, the immunoglobulin polymerization is thought to be associated with higher affinity or binding strength for antigens than monomeric IgG with the same specificities.73,74 This consideration was also relevant to influenza virus-specific sIgA in the RT, because the polymeric sIgA was more cross-reactive with a several-fold higher activity in hemagglutination inhibition and virus neutralization than monomeric IgG.75 In line with these observations, the cross-reactivity of the sIgA was stronger than serum IgG, in mice immunized with the pdmH1N1 CAIV. It was demonstrated that while the cross-reactive serum IgG titers were only a tenth of the homologous strain-specific IgG titers, the sIgA titers in the RT were similar across homologous and heterologous viruses including seasonal and H5 influenza strains.40
Although neutralizing antibodies are recognized as primary effectors responsible for successful protection against homologous infection, there is also a wealth of evidence that non-neutralizing antibodies are intimately associated with protection, especially against heterologous infections. While the NP-specific CTL responses were recognized to be one of the responsible factors for heterosubtypic resistance, the involvement of NP-specific antibodies in protection has largely been disregarded. This was not because of the absence of the NP-specific antibody but because of its relatively weak contribution to overall protection because the NP-specific antibodies provided only partial protection when passively transferred into naïve mice.76 In another study, the non-neutralizing antibodies by themselves did not provide any protection to heterologous challenge in animal model, however they reduced the morbidity and promoted recovery from the infection in cooperation with memory T cells.77 Intriguingly, the non-neutralizing antibodies were also found to facilitate the expansion of responding memory CD8 T cells, which suggests the coordination of B cells and T cells in eliciting the cross-protective immunity.77 Other protective mechanisms by the non-neutralizing antibodies involve either macrophages or natural killer (NK) cells. IgG antibodies bound to a virus particle are recognized by Fc receptors expressed on the macrophages, which then actively ingest the opsonized virus, playing a pivotal role in the clearance of the infection.78 However, the NK cells were reported to recognize antibodies bound to the influenza M2 proteins expressed on the surface of virus-infected cells and this finally lead to cell death also known as the antibody-dependent cell-mediated cytotoxicity.79 These mechanisms may explain the existence of the non-neutralizing antibodies found in the serum of CAIV-immunized animals that yielded a considerable level of IgG antibody titers in ELISA, while not being detected in HI assay nor in VN assay.40
From the late 2000s, the highly variable surface glycoprotein, HA, has become the focus in identifying conserved regions in the protein and their corresponding antibodies expected to be cross-reactive, with the aim of developing universal vaccine constructs. These successful findings not only provide a promising avenue for novel vaccine strategy, but also illuminate an additional contributing factor to the cross-protection conferred by the CAIVs. Two conserved regions in the HA were responsible for recognition by newly discovered antibodies - the membrane-proximal stem domain harboring the membrane fusion peptide80-82 and the receptor binding pocket in the globular head domain.83,84 Monoclonal antibodies that recognized each of these two domains provided heterologous immunity when passively transferred into naive mice. These findings also open the possibility of significantly broadening the cross-reactivity of the influenza live vaccines through rational design. This may be possible, for example, via appropriate genetic engineering of the vaccine such that the exposure of the conserved domains of the HA could be enhanced while maintaining other desirable characters. Recently, the structural and genetic basis for such broadly neutralizing antibodies was identified as largely originating from a specific antibody gene, heavy-chain variable region IGHV1-69.65,85 These reports could invigorate further discoveries of hitherto unknown antibodies derived from the antibody gene.
All of the principles of cross-protection explained above are results from adaptive immune responses acquired mainly after viral infection or vaccination. While adaptive immunity is highly specific to a particular pathogen or its products, innate immunity provides immediate protection against a wide range of pathogens, although the protection is partial and short-lived.67 Innate immunity involves the induction of antiviral interferon responses and pro-inflammatory cytokines and chemokines, which help healthy cells resist pathogens including viruses.67 It has been reported that cold-adapted X-31 (A/H3N2) donor strain provided 100% protection in mice when the vaccine was inoculated three or four days prior to a lethal challenge with either heterosubtypic (A/New Caledonia/99, H1N1) or even a heterotypic (B/Shangdong/97) strain.86 The immediate and broad spectrum of protection was shown to be mediated by innate immunity rather than by specific antibodies. The innate immunity by the CAIVs seems to be beneficial particularly in the event of an unexpected and sudden emergence of an influenza pandemic of a completely new subtype when there is no sufficient time for the production of a matching vaccine. Therefore, the CAIVs provide an immediate and broad-spectrum protection against various influenza strains, which may extend and complement the current influenza control strategies.
As discussed above, a potential drawback associated with the annual use of inactivated vaccines in children comprises a preferential and skewed induction of the humoral responses over the CTLs, raising the concern of decreased cross-protective immunity.87-89 Conversely, an imbalance within the influenza virus-specific CTLs was also recognized as a potentially pathogenic factor leading to the unexpected loss of homologous protective immunity by an exaggerated expansion of the cross-reactive but less protective CTLs. Direct evidence for the existence of such phenomenon has not yet been presented for the influenza virus. Considering that the CAIVs induce strong CTL responses, it is worth discussing closely related cases relevant to the rational design of a more broadly-protective live vaccine without any pathogenic effects.
Of the many virus-derived peptides processed and presented, only a few stimulate strong CD8 T cell responses, which means that a hierarchy exists of dominance in the T cell responses, with some peptides recognized strongly (immunodominant), some weakly (subdominant), and others only in the absence of the dominant peptides (cryptic) (Fig. 1).90 It was previously proposed that the hierarchy of immunodominance shaped by previous exposure to a pathogen varies markedly upon a secondary exposure to a heterologous strain. T cells specific to cross-reactive epitopes shared by the two pathogens dominate both the primary and memory pool while those specific for non-cross-reactive epitopes are selectively lost.21 This alteration in the immunodominance hierarchy was described for influenza viruses as well, which lead to a selective expansion of cross-reactive T cells upon subsequent infection with a heterosubtypic influenza strain.91 This raises a reasonable question as to why most people become susceptible to influenza variants circulating in a subsequent season despite repeated prior exposures to the viruses. The most plausible answer to this question may be that, without the help of neutralizing antibody responses, the cross-reactive T cell responses by themselves do not provide sufficient protection against the heterologous influenza strains. This consideration provides strong support towards vaccination with the CAIV because it would boost the underlying cross-reactive T cell responses and induce protective neutralizing antibody responses as well.
Despite the potential advantages of selectively expanding cross-reactive T cell responses by vaccination, serious concerns were also raised by other publications on potentially pathogenic effects of the cross-reactive T cell epitopes. For example, herpes simplex virus-1 and theiler's virus were found to stimulate the autoreactive T cells that target the proteins of the eye and brain, leading to conjunctivitis and encephalitis, respectively.92,93 Although there is no reported evidence yet for the existence of self-reactive T cell epitopes in influenza viral peptides, caution should be taken when selecting and modulating the cross-reactive epitopes for generating more cross-protective live vaccines.
Furthermore, it was shown that if the cross-reactive but less protective CTL response was selectively expanded by subsequent heterologous infections, then the pre-existing normally protective T cell response would become markedly suppressed leading to the attrition of protective immunity to the previous homologous strain.94 Considering that the CTL responses alone fail to support the protection against the influenza viruses, such cross-reactive pathogenic epitopes may raise concern, especially for individuals with pre-existing strong cross-reactive T cells by repeated exposures to the viruses but with poor neutralizing antibodies. In those individuals, vaccination may aggravate, rather than alleviate, the symptoms from the infection. This consideration emphasizes the importance of neutralizing activity for successful protection against the influenza viruses and further suggests that a rational design of influenza live vaccines should focus primarily on its ability to induce neutralizing antibodies against the HA and NA required to prevent infection in the first place, and secondarily on increasing the cross-protection.
A hugely diverse antigenicity and the continual evolution of influenza viruses through antigenic drift and antigenic shift represent the biggest challenge to the development of a long lasting and broadly protective vaccine. The CAIVs appear better fit to the concept of cross-protection than inactivated vaccines for several reasons discussed above. In addition to making better use of previously known mechanisms, further discovery and appropriate modulation of novel cross-protective T cell epitopes would greatly improve the protection coverage of the vaccines. Likewise, it will be worthwhile to generate diverse strains of cold-adapted donor strains because each different backbone strain would differentially influence the immune responses both in quantity and quality, particularly through the CTL responses directed to internal viral proteins of the donor strain.
Although some of the principles underlying the cross-protection were extrapolated from previous reports dealing with inactivated vaccines or experimental infections, it is reasonable to assume that factors uncovered from those studies may also contribute to the cross-protection afforded by the live vaccines. In addition to the cold-adaptation, several attempts were made to develop alternative live vaccine strategies by reverse genetics technology.67 With varying degrees depending on their attenuation mechanisms, they will also stimulate a similar repertoire of immunological correlates responsible for cross-protection. However, this does not imply that live vaccines always provide the best options for inducing the highest level of cross-protection. More specialized regimens such as DNA vaccines or other viral vectored vaccines encoding multiple conserved epitopes under a strong promoter should also be considered.
Many studies on cross-protection using infection models or vaccinations revealed a huge complexity of the immune system, ranging from the coordination of multiple cellular and humoral components required for cross-protection, to the T cell immunodominance that in some cases may exert some harmful effects through autoimmunity or the loss of protective immunity by exaggerated expansion of cross-reactive but less protective CTL responses. These findings underscore a more detailed understanding of our immune system and the importance of predicting the evolution of the influenza viruses for the rational design of the universal influenza vaccines.
Figures and Tables
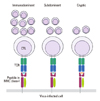 | Fig. 1T cell Immunodominance. Virus-specific CTLs recognize the complex of the viral peptide and MHC class-I molecule presented on the membrane of the infected cells. Some peptides generate strong signals and thereby lead to the robust clonal expansion of the responding CTLs (immunodominant), some generate weak (subdominant) signals, and others barely generate a signal (cryptic) only detectable in the absence of the others. The hierarchy of the T cell immunodominance shaped by primary exposure to a virus varies upon a subsequent infection with heterologous virus. CTL, cytotoxic T lymphocyte; TCR, T cell receptor; MHC, major histocompatiblity complex. |
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea CDC (2009-E00522-00). This study was also supported in part by the National Research Foundation of the Korean Government (MEST) (2011-0001246).
References
1. Englund JA, Champlin RE, Wyde PR, Kantarjian H, Atmar RL, Tarrand J, et al. Common emergence of amantadine- and rimantadine-resistant influenza A viruses in symptomatic immunocompromised adults. Clin Infect Dis. 1998. 26:1418–1424.

2. de Jong MD, Tran TT, Truong HK, Vo MH, Smith GJ, Nguyen VC, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005. 353:2667–2672.

3. Wiley DC, Wilson IA, Skehel JJ. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981. 289:373–378.

4. de Jong JC, Claas EC, Osterhaus AD, Webster RG, Lim WL. A pandemic warning? Nature. 1997. 389:554.

5. Claas EC, Osterhaus AD, van Beek R, De Jong JC, Rimmelzwaan GF, Senne DA, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998. 351:472–477.

6. Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998. 279:393–396.

7. Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012. 336:1534–1541.

8. Russell CA, Fonville JM, Brown AE, Burke DF, Smith DL, James SL, et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science. 2012. 336:1541–1547.

9. Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012. 486:420–428.

10. Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009. 325:197–201.
11. Shinde V, Bridges CB, Uyeki TM, Shu B, Balish A, Xu X, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005-2009. N Engl J Med. 2009. 360:2616–2625.

12. Octaviani CP, Ozawa M, Yamada S, Goto H, Kawaoka Y. High level of genetic compatibility between swine-origin H1N1 and highly pathogenic avian H5N1 influenza viruses. J Virol. 2010. 84:10918–10922.
13. Cline TD, Karlsson EA, Freiden P, Seufzer BJ, Rehg JE, Webby RJ, et al. Increased pathogenicity of a reassortant 2009 pandemic H1N1 influenza virus containing an H5N1 hemagglutinin. J Virol. 2011. 85:12262–12270.

14. Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010. 235:244–266.

15. Bender BS, Croghan T, Zhang L, Small PA Jr. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992. 175:1143–1145.

16. Doherty PC. Cytotoxic T cell effector and memory function in viral immunity. Curr Top Microbiol Immunol. 1996. 206:1–14.

17. Price GE, Ou R, Jiang H, Huang L, Moskophidis D. Viral escape by selection of cytotoxic T cell-resistant variants in influenza A virus pneumonia. J Exp Med. 2000. 191:1853–1867.

18. McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983. 309:13–17.

19. Selin LK, Vergilis K, Welsh RM, Nahill SR. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J Exp Med. 1996. 183:2489–2499.

20. Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, et al. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999. 11:733–742.
21. Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002. 3:627–634.

22. Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, O'Brien D, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001. 19:1732–1737.

23. Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001. 357:1937–1943.

24. Aymard M, Valette M, Lina B, Thouvenot D. Surveillance and impact of influenza in Europe. Groupe Regional d'Observation de la Grippe and European Influenza Surveillance Scheme. Vaccine. 1999. 17:Suppl 1. S30–S41.
25. Klimov A, Simonsen L, Fukuda K, Cox N. Surveillance and impact of influenza in the United States. Vaccine. 1999. 17:Suppl 1. S42–S46.

26. Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, et al. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010. 1:pii: e00018-10.

27. Ulmer JB, Fu TM, Deck RR, Friedman A, Guan L, DeWitt C, et al. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol. 1998. 72:5648–5653.

28. Fu TM, Guan L, Friedman A, Schofield TL, Ulmer JB, Liu MA, et al. Dose dependence of CTL precursor frequency induced by a DNA vaccine and correlation with protective immunity against influenza virus challenge. J Immunol. 1999. 162:4163–4170.
29. Ulmer JB, Deck RR, Dewitt CM, Donnhly JI, Liu MA. Generation of MHC class I-restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology. 1996. 89:59–67.

30. Klinman DM, Takeno M, Ichino M, Gu M, Yamshchikov G, Mor G, et al. DNA vaccines: safety and efficacy issues. Springer Semin Immunopathol. 1997. 19:245–256.

31. Bodewes R, Fraaij PL, Geelhoed-Mieras MM, van Baalen CA, Tiddens HA, van Rossum AM, et al. Annual vaccination against influenza virus hampers development of virus-specific CD8+ T cell immunity in children. J Virol. 2011. 85:11995–12000.

32. Bodewes R, Kreijtz JH, Baas C, Geelhoed-Mieras MM, de Mutsert G, van Amerongen G, et al. Vaccination against human influenza A/H3N2 virus prevents the induction of heterosubtypic immunity against lethal infection with avian influenza A/H5N1 virus. PLoS One. 2009. 4:e5538.

33. Bodewes R, Kreijtz JH, Geelhoed-Mieras MM, van Amerongen G, Verburgh RJ, van Trierum SE, et al. Vaccination against seasonal influenza A/H3N2 virus reduces the induction of heterosubtypic immunity against influenza A/H5N1 virus infection in ferrets. J Virol. 2011. 85:2695–2702.

34. Bodewes R, Kreijtz JH, Hillaire ML, Geelhoed-Mieras MM, Fouchier RA, Osterhaus AD, et al. Vaccination with whole inactivated virus vaccine affects the induction of heterosubtypic immunity against influenza virus A/H5N1 and immunodominance of virus-specific CD8+ T-cell responses in mice. J Gen Virol. 2010. 91(Pt 7):1743–1753.

35. Suguitan AL Jr, McAuliffe J, Mills KL, Jin H, Duke G, Lu B, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006. 3:e360.

36. Fan S, Gao Y, Shinya K, Li CK, Li Y, Shi J, et al. Immunogenicity and protective efficacy of a live attenuated H5N1 vaccine in nonhuman primates. PLoS Pathog. 2009. 5:e1000409.
37. Desheva JA, Lu XH, Rekstin AR, Rudenko LG, Swayne DE, Cox NJ, et al. Characterization of an influenza A H5N2 reassortant as a candidate for live-attenuated and inactivated vaccines against highly pathogenic H5N1 viruses with pandemic potential. Vaccine. 2006. 24:6859–6866.

38. Chen GL, Min JY, Lamirande EW, Santos C, Jin H, Kemble G, et al. Comparison of a live attenuated 2009 H1N1 vaccine with seasonal influenza vaccines against 2009 pandemic H1N1 virus infection in mice and ferrets. J Infect Dis. 2011. 203:930–936.

39. Yang P, Duan Y, Wang C, Xing L, Gao X, Tang C, et al. Immunogenicity and protective efficacy of a live attenuated vaccine against the 2009 pandemic A H1N1 in mice and ferrets. Vaccine. 2011. 29:698–705.

40. Jang YH, Byun YH, Lee YJ, Lee YH, Lee KH, Seong BL. Cold-adapted pandemic 2009 H1N1 influenza virus live vaccine elicits cross-reactive immune responses against seasonal and H5 influenza A viruses. J Virol. 2012. 86:5953–5958.

41. Murphy BR, Clements ML. The systemic and mucosal immune response of humans to influenza A virus. Curr Top Microbiol Immunol. 1989. 146:107–116.

42. Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001. 75:5141–5150.

43. Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002. 20:1340–1353.

44. Powers DC, Fries LF, Murphy BR, Thumar B, Clements ML. In elderly persons live attenuated influenza A virus vaccines do not offer an advantage over inactivated virus vaccine in inducing serum or secretory antibodies or local immunologic memory. J Clin Microbiol. 1991. 29:498–505.

45. Hillaire ML, Osterhaus AD, Rimmelzwaan GF. Induction of virus-specific cytotoxic T lymphocytes as a basis for the development of broadly protective influenza vaccines. J Biomed Biotechnol. 2011. 2011:939860.

46. Belshe RB, Gruber WC, Mendelman PM, Cho I, Reisinger K, Block SL, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000. 136:168–175.

47. Nichol KL, Mendelman PM, Mallon KP, Jackson LA, Gorse GJ, Belshe RB, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999. 282:137–144.

48. Clover RD, Crawford S, Glezen WP, Taber LH, Matson CC, Couch RB. Comparison of heterotypic protection against influenza A/Taiwan/86 (H1N1) by attenuated and inactivated vaccines to A/Chile/83-like viruses. J Infect Dis. 1991. 163:300–304.

49. Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994. 169:68–76.

50. Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006. 355:2513–2522.

51. Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007. 356:685–696.

52. Powell TJ, Strutt T, Reome J, Hollenbaugh JA, Roberts AD, Woodland DL, et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007. 178:1030–1038.

53. Ding H, Tsai C, Zhou F, Buchy P, Deubel V, Zhou P. Heterosubtypic antibody response elicited with seasonal influenza vaccine correlates partial protection against highly pathogenic H5N1 virus. PLoS One. 2011. 6:e17821.

54. Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007. 4:e59.

55. van Maurik A, Sabarth N, Dacho HS, Brühl P, Schwendinger M, Crowe BA, et al. Seasonal influenza vaccine elicits heterosubtypic immunity against H5N1 that can be further boosted by H5N1 vaccination. Vaccine. 2010. 28:1778–1785.

56. Shi J, Wen Z, Guo J, Zhang Y, Deng G, Shu Y, et al. Protective efficacy of an H1N1 cold-adapted live vaccine against the 2009 pandemic H1N1, seasonal H1N1, and H5N1 influenza viruses in mice. Antiviral Res. 2012. 93:346–353.

57. WHO/OIE/FAO H5N1 Evolution Working Group. Continued evolution of highly pathogenic avian influenza A (H5N1): updated nomenclature. Influenza Other Respi Viruses. 2012. 6:1–5.
58. WHO/OIE/FAO H5N1 Evolution Working Group. Continuing progress towards a unified nomenclature for the highly pathogenic H5N1 avian influenza viruses: divergence of clade 2.2 viruses. Influenza Other Respi Viruses. 2009. 3:59–62.
59. Gustin KM, Maines TR, Belser JA, van Hoeven N, Lu X, Dong L, et al. Comparative immunogenicity and cross-clade protective efficacy of mammalian cell-grown inactivated and live attenuated H5N1 reassortant vaccines in ferrets. J Infect Dis. 2011. 204:1491–1499.

60. Chen Z, Wang W, Zhou H, Suguitan AL Jr, Shambaugh C, Kim L, et al. Generation of live attenuated novel influenza virus A/California/7/09 (H1N1) vaccines with high yield in embryonated chicken eggs. J Virol. 2010. 84:44–51.

61. Girard MP, Katz JM, Pervikov Y, Hombach J, Tam JS. Report of the 7th meeting on Evaluation of Pandemic Influenza Vaccines in Clinical Trials, World Health Organization, Geneva, 17-18 February 2011. Vaccine. 2011. 29:7579–7586.

62. Chen GL, Lau YF, Lamirande EW, McCall AW, Subbarao K. Seasonal influenza infection and live vaccine prime for a response to the 2009 pandemic H1N1 vaccine. Proc Natl Acad Sci U S A. 2011. 108:1140–1145.

63. Sun K, Ye J, Perez DR, Metzger DW. Seasonal FluMist vaccination induces cross-reactive T cell immunity against H1N1 (2009) influenza and secondary bacterial infections. J Immunol. 2011. 186:987–993.

64. Qiu C, Huang Y, Wang Q, Tian D, Zhang W, Hu Y, et al. Boosting heterosubtypic neutralization antibodies in recipients of 2009 pandemic H1N1 influenza vaccine. Clin Infect Dis. 2012. 54:17–24.

65. Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011. 208:181–193.

66. Jang YH, Seong BL. Principles underlying rational design of live attenuated influenza vaccines. Clin Exp Vaccin Res. 2012. 1:35–49.

67. Murphy KM, Travers P, Walport M, Janeway C. Janeway's Immunobiology. 2008. 7th ed. Garland Science.
68. Yewdell JW, Bennink JR, Smith GL, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985. 82:1785–1789.

69. Gotch F, McMichael A, Smith G, Moss B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J Exp Med. 1987. 165:408–416.

70. Tan PT, Khan AM, August JT. Highly conserved influenza A sequences as T cell epitopes-based vaccine targets to address the viral variability. Hum Vaccin. 2011. 7:402–409.

71. Gras S, Kedzierski L, Valkenburg SA, Laurie K, Liu YC, Denholm JT, et al. Cross-reactive CD8+ T-cell immunity between the pandemic H1N1-2009 and H1N1-1918 influenza A viruses. Proc Natl Acad Sci U S A. 2010. 107:12599–12604.

72. Grandea AG 3rd, Olsen OA, Cox TC, Renshaw M, Hammond PW, Chan-Hui PY, et al. Human antibodies reveal a protective epitope that is highly conserved among human and nonhuman influenza A viruses. Proc Natl Acad Sci U S A. 2010. 107:12658–12663.

73. Hendrickson BA, Conner DA, Ladd DJ, Kendall D, Casanova JE, Corthesy B, et al. Altered hepatic transport of immunoglobulin A in mice lacking the J chain. J Exp Med. 1995. 182:1905–1911.

74. Niles MJ, Matsuuchi L, Koshland ME. Polymer IgM assembly and secretion in lymphoid and nonlymphoid cell lines: evidence that J chain is required for pentamer IgM synthesis. Proc Natl Acad Sci U S A. 1995. 92:2884–2888.

75. Renegar KB, Jackson GD, Mestecky J. In vitro comparison of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J Immunol. 1998. 160:1219–1223.
76. Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol. 2008. 181:4168–4176.

77. Rangel-Moreno J, Carragher DM, Misra RS, Kusser K, Hartson L, Moquin A, et al. B cells promote resistance to heterosubtypic strains of influenza via multiple mechanisms. J Immunol. 2008. 180:454–463.

78. Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001. 166:7381–7388.

79. Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004. 172:5598–5605.

80. Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009. 324:246–251.

81. Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011. 333:850–856.

82. Prabhu N, Prabakaran M, Ho HT, Velumani S, Qiang J, Goutama M, et al. Monoclonal antibodies against the fusion peptide of hemagglutinin protect mice from lethal influenza A virus H5N1 infection. J Virol. 2009. 83:2553–2562.

83. Krause JC, Tsibane T, Tumpey TM, Huffman CJ, Basler CF, Crowe JE Jr. A broadly neutralizing human monoclonal antibody that recognizes a conserved, novel epitope on the globular head of the influenza H1N1 virus hemagglutinin. J Virol. 2011. 85:10905–10908.

84. Whittle JR, Zhang R, Khurana S, King LR, Manischewitz J, Golding H, et al. Broadly neutralizing human antibody that recognizes the receptor-binding pocket of influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 2011. 108:14216–14221.
85. Lingwood D, McTamney PM, Yassine HM, Whittle JR, Guo X, Boyington JC, et al. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature. 2012. 489:566–570.

86. Seo SU, Lee KH, Byun YH, Kweon MN, Seong BL. Immediate and broad-spectrum protection against heterologous and heterotypic lethal challenge in mice by live influenza vaccine. Vaccine. 2007. 25:8067–8076.

87. Bodewes R, Kreijtz JH, Rimmelzwaan GF. Yearly influenza vaccinations: a double-edged sword. Lancet Infect Dis. 2009. 9:784–788.

89. Skowronski DM, De Serres G, Crowcroft NS, Janjua NZ, Boulianne N, Hottes TS, et al. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada. PLoS Med. 2010. 7:e1000258.

90. Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999. 17:51–88.

91. Haanen JB, Wolkers MC, Kruisbeek AM, Schumacher TN. Selective expansion of cross-reactive CD8(+) memory T cells by viral variants. J Exp Med. 1999. 190:1319–1328.

92. Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998. 279:1344–1347.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download