Abstract
A 42-year-old man was involved in a motor vehicle collision. Imaging studies revealed the presence of a post-traumatic aortic pseudo-aneurysm (about 34×26 cm) arising from the descending thoracic aorta at the level of the left subclavian artery (LSA), prone to rupture. Thoracic endovascular aneurysm repair (TEVAR) was the only feasible option due to his poor overall medical status. In this case, LSA needed to be covered in order to extend the proximal landing zone. Eventually, modified TEVAR was successfully performed by means of the chimney technique to preserve flow to the LSA and to prevent flow into the pseudoaneurysmal sac.
Pseudoaneurysm after trauma is an out-pouching of the blood vessel, resulting from a deficiency in the tunica media and intima. The risk of rupture is high, and surgery has traditionally been recommended in all patients with thoracic aortic pseudoaneurysm irrespective of symptom status.1 Thoracic endovascular aneurysm repair (TEVAR) is an alternative to open surgery for patients with the significant risk of surgery,2,3 however, the lack of adequate landing zones for stent grafts, and close proximity to supra-aortic branches make effective and safe delivery of endovascular therapy technically challenging. We describe a case of successful TEVAR with the chimney technique for blunt traumatic pseudoaneurysm of the aortic arch in a no-option patient.
42-year-old man was involved in a motor vehicle collision. On arrival, he was hypotensive and tachycardic. Further diagnostic work-up revealed multiple injuries including suspected intra-peritoneal hemorrhage, and multiple pelvic bone fracture. A computed tomography (CT) scan showed the presence of a post-traumatic aortic pseudo-aneurysm (about 34×26 cm) arising from the descending thoracic aorta (DTA). The neck was located immediately after the origin of the left subclavian artery (LSA) on the lesser curvature of the aorta (Fig. 1). Because of his poor overall medical status, difficulty of maintaining low blood pressure, and suspected coagulopathy, TEVAR remained the only feasible option. But, the presence of an inadequate proximal landing zone was an important limiting for endovasucular repair. LSA needed to be covered in order to extend the proximal landing zone, improve fixation and achieve an adequate seal. Therefore, modified TEVAR was planned by means of the chimney technique to preserve flow to the LSA and to prevent flow into the pseudoaneurysmal sac. Thus, left common femoral artery (CFA) was prepared with the preclosing technique using two closure devices (ProGlide, Abbott Vascular Devices, Redwood, CA, USA) in an off-label fashion by deploying the devices before insertion of the 18 Fr sheath (UltimumTM EV, St. Jude, Minnetonka, MN, USA). For the chimney technique of the LSA, the left brachial artery was punctured with a 7-F sheath, and the chimney stent-SMART-Control stent (Cordis; Johnson & Johnson Medical, Miami, FL, USA) of 10 mm diameter and 40 mm in length was delivered in advance into the LSA, with a short segment protruding into the aortic arch lumen. For TEVAR, the diameter of the endoluminal graft was based on the diameter of the true lumen-aortic arch and the healthy aorta just before the lesion with an oversizing of about 10-20% to permit adequate proximal seal and fixation. With injection contrast medium for visualizing the position of the stent graft with a 5 F pigtail catheter via right femoral access in the ascending aorta, a Thoracic SEAL stent graft (S&G Bio Tech CO., Seongnam, Korea) made of 34-30 mm tapered diameter and 110 mm length was deployed. The landmark for stent graft placement was the origin of the left common carotid artery (CCA). Next, the chimney stent was deployed in the LSA (Fig. 2). Completion angiography revealed widely patent LSA and left CCA, exclusive of the pseudoaneurysm without evidence of endoleak. Then, the left CFA was closed with preformed knots of two sutures of closure devices. A follow-up CT performed after 7 days indicated no proximal endoleak with complete exclusion of the thoracic aortic pseudoaneurysm, as well as free flow into the LSA and left CCA (Fig. 3).
TEVAR is difficult and problematic when the aortic arch is involved. Because the main challenge lies in maintaining blood flow to the vital supra-aortic branches while the arch is being covered by the stent graft, combined stent-grafting and surgery with extra-anatomic bypass grafts could be an excellent approach in such cases.4,5 However, these techniques also obviate the need for an open surgical approach and are not suitable for urgent cases. Thoracic aortic pseudoaneruysm is a rare and potentially fatal problem.6 A perfused pseudoaneurysm may partially clot and organize with a fibrous wall potentially evolving into a large pseudoaneurysm which is prone to rupture.7 In our patient, TEVAR remained the only feasible option. The main challenge was that intentional covering of the LSA needed to extend the proximal landing zone and achieve an adequate seal. A few studies report that intentional coverage of the LSA without revascularization is not associated with additional morbidity, whereas many studies report a higher incidence of postoperative arm ischemia and posterior circulation strokes compared with patients who did not undergo intentional coverage.8-10 We, therefore, aimed to perform the chimney technique, which is based on previously reported intentional LSA.11,12 The chimney device is defined as a bare or covered stent graft that is implanted parallel to the main aortic stent graft and extends into an over-stented vital side branch in order to preserve its perfusion.11 The chimney technique was originally introduced by Greenberg, et al.,13 who used renal stents to depress the proximal edge of stent-graft fabric that protruded a few millimeters above the renal artery ostium. Since then, it has been used for preservation of vital supra aortic branches during TEVAR.14,15 For the chimney device type, a covered stent graft would seem to provide a better seal than a bare stent. However, Feng, et al.16 state that covered stent grafts offer no additional benefits, except that a long channel is required for the branch. Few data are available concerning the superiority and matching of different chimney devices. We, therefore, believe that any type of chimney device, readily available in any interventional suite, can be used to form the chimney. Also, our team believes that self-expanding stent also has sufficient crush resistance and remains open after placement.
To our knowledge, this is the first report to show that traumatic aortic arch pseudoaneurysm after blunt trauma with intra-peritoneal hemorrhage and multiple bone fractures was successfully treated by TEVAR with chimney technique. Long term results are needed before this technique can widely be applied. However, it seems to be an alternative treatment option in urgent-traumatic pseudoaneruysm, and can be used in rescue procedures to salvage aortic side branches over-stented during TEVAR.
Figures and Tables
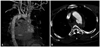 | Fig. 1Pseudoaneurysm and its relationship with surrounding structures. (A) Saccular pseudoaneurysm was noted just distal to the orifice of the LSA on the lesser curvature of the aorta on the three-dimensional reconstruction image of the CT. (B) Axial CT scan showed the presence of a post-traumatic aortic pseudo-aneurysm (about 34×26 cm) arising from the DTA at the level of the LSA. LSA, left subclavian artery; CT, computed tomography; DTA, descending thoracic aorta. |
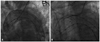 | Fig. 2Endograft deployment resulted in near-total coverage of the LSA (A), followed by retrograde chimney stenting of the LSA origin via the trans-brachial approach (B). The landmark for stent graft placement was the origin of the left CCA. Note that the chimney stent was delivered in advance into the LSA, with protruding into the aortic arch lumen. LSA, left subclavian artery; CCA, common carotid artery. |
References
1. Alla VM, Suryanarayana PG, Thambidorai SK. Thoracic aortic pseudoaneurysm following noncardiovascular surgery: a rare complication that can mimic common chest emergencies. South Med J. 2010. 103:1186–1188.

2. Chaikof EL, Mutrie C, Kasirajan K, Milner R, Chen EP, Veeraswamy RK, et al. Endovascular repair for diverse pathologies of the thoracic aorta: an initial decade of experience. J Am Coll Surg. 2009. 208:802–816.

3. Kpodonu J, Wheatley GH 3rd, Ramaiah VG, Rodriguez-Lopez JA, Strumpf RK, Diethrich EB. Endovascular repair of an ascending aortic pseudoaneurysm with a septal occluder device: mid-term follow-up. Ann Thorac Surg. 2008. 85:349–351.

5. Kang WC, Shin EK, Ahn TH, Lee KH, Moon CI, Han SH, et al. Combined open and endovascular repair for aortic arch pathology. Korean Circ J. 2010. 40:399–404.

6. Razzouk A, Gundry S, Wang N, Heyner R, Sciolaro C, Van Arsdell G, et al. Pseudoaneurysms of the aorta after cardiac surgery or chest trauma. Am Surg. 1993. 59:818–823.
7. Bizzarri F, Mattia C, Ricci M, Chirichilli I, Santo C, Rose D, et al. Traumatic aortic arch false aneurysm after blunt chest trauma in a motocross rider. J Cardiothorac Surg. 2008. 3:23.

8. Woo EY, Carpenter JP, Jackson BM, Pochettino A, Bavaria JE, Szeto WY, et al. Left subclavian artery coverage during thoracic endovascular aortic repair: a single-center experience. J Vasc Surg. 2008. 48:555–560.

9. Peterson BG, Eskandari MK, Gleason TG, Morasch MD. Utility of left subclavian artery revascularization in association with endoluminal repair of acute and chronic thoracic aortic pathology. J Vasc Surg. 2006. 43:433–439.

10. Reece TB, Gazoni LM, Cherry KJ, Peeler BB, Dake M, Matsumoto AH, et al. Reevaluating the need for left subclavian artery revascularization with thoracic endovascular aortic repair. Ann Thorac Surg. 2007. 84:1201–1205.

11. Sugiura K, Sonesson B, Akesson M, Björses K, Holst J, Malina M. The applicability of chimney grafts in the aortic arch. J Cardiovasc Surg (Torino). 2009. 50:475–481.
12. Ohrlander T, Sonesson B, Ivancev K, Resch T, Dias N, Malina M. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther. 2008. 15:427–432.

13. Greenberg RK, Clair D, Srivastava S, Bhandari G, Turc A, Hampton J, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003. 38:990–996.

14. Criado FJ. A percutaneous technique for preservation of arch branch patency during thoracic endovascular aortic repair (TEVAR): retrograde catheterization and stenting. J Endovasc Ther. 2007. 14:54–58.

15. Hiramoto JS, Schneider DB, Reilly LM, Chuter TA. A double-barrel stent-graft for endovascular repair of the aortic arch. J Endovasc Ther. 2006. 13:72–76.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download