Abstract
Purpose
Cell transplantation of myelin-producing exogenous cells is being extensively explored as a means of remyelinating axons in X-linked adrenoleukodystrophy. We determined whether 3,3',5-Triiodo-L-thyronine (T3) overexpresses the ABCD2 gene in the polysialylated (PSA) form of neural cell adhesion molecule (NCAM)-positive cells and promotes cell proliferation and favors oligodendrocyte lineage differentiation.
Materials and Methods
PSA-NCAM+ cells from newborn Sprague-Dawley rats were grown for five days on uncoated dishes in defined medium with or without supplementation of basic fibroblast growth factor (bFGF) and/or T3. Then, PSA-NCAM+ spheres were prepared in single cells and transferred to polyornithine/fibronectin-coated glass coverslips for five days to determine the fate of the cells according to the supplementation of these molecules. T3 responsiveness of ABCD2 was analyzed using real-time quantitative polymerase chain reaction, the growth and fate of cells were determined using 5-bromo-2-deoxyuridine incorporation and immunocytochemistry, respectively.
X-linked adrenoleukodystrophy (ALD), a fatal neurodegenerative disorder, is caused by a defect in the ATP-binding Cassette Sub-family D Member 1 (ABCD1) gene, which is involved in the peroxisomal oxidation of very long chain fatty acids (VLCFAs).1
Inflammatory demyelination is known to be a predominant pathological change in X-ALD. Currently, the therapeutic options of ALD patients are extremely limited.1 Dietary therapy with Lorenzo's oil found to reduce the VLCFA levels in plasma but not in the brain and does not retard the progressive demyelination. To date, there are no suitable therapeutic drugs available that can cure X-ALD either. Although allogeneic hematopoietic stem cell transplantation is the only effective therapy for childhood cerebral adrenoleukodystrophy, limitations by HLA-matched donors, a considerable risk of mortality, and a narrow therapeutic window remain to be resolved. More recently, clinical trial of genetically corrected autologous hematopoietic stem cells in ALD patients was conducted.2 However, affected cerebral lesions are barely recovered and their long term efficacy needs to be proven.
Cell transplantation of myelin-producing exogenous cells is being extensively explored as a means of replacing the genetically dysfunctional cells.3 Precursors of oligodendrocyte (OD) progenitors express the polysialylated (PSA) form of the neural cell adhesion molecule (NCAM) and these PSA-NCAM+ cells are useful for transplantation studies because of their high motility, rapid proliferation and default differentiation to glial cells within the brain.4,5 In rodents, 3,3',5-triiodo-L-thyronine (T3) can enhance PSA-NCAM+ cell growth in the presence of basic fibroblast growth factor (bFGF), favoring an OD fate.6 In addition, T3 is suggested to induce the ABCD2 redundant gene which is functionally related to ABCD1 gene and can correct the biochemical defect in the ABCD1 knockout mouse.7,8
Here, we determined if the emergence of PSA-NCAM at the surface of neonatal rat brain precursors coincides with their restriction to a mostly glial fate. T3 modulates these events by enhancing PSA-NCAM+ cell growth in bFGF and favoring an OD fate. We investigated the regulation of ABCD2 gene expression in PSA-NCAM+ cells upon T3 treatment.
Newborn Sprague-Dawley rat pups were obtained from Charles River Laboratories (Wilmington, MA, USA). DMEM/F12 and B27 purchased from Invitrogen (Grand Island, NY, USA). Percoll was obtained from Pharmacia (Uppsala, Sweden). All other products were purchased from Sigma Chemicals (St. Louis, MO, USA) if not specified otherwise. The protocol used in this study was approved by the Yonsei University Animal Use Committee and by the IRB for animal research.
PSA-NCAM+ pre-progenitors were obtained by a Percoll density gradient using a slight modification of the isolation procedure described by Lubetzki and co-workers (Fig. 1A).6,9 In brief, the Sprague-Dawley newborn rats were sacrificed by decapitation and their brains were prepared after olfactory bulbs, cerebellum, and brainstem were removed. The brains that were minced in Hanks' medium (18 mM sodium bicarbonate) were enzymatically digested with 0.1% trypsin/EDTA during 15 minutes at 37℃ and digestion was stopped by fetal bovine serum. The tissue was filtered using nylon meshes with 70 and 40 µm pores. Dissociated cells were layered on a Percoll density gradient consisting of 4.8 mL of 90% Percoll in 0.15 M NaCl, and 7.2 mL of 1X HBSS, and centrifuged at 23500 g for 45 minutes at 4℃. The fraction was collected and washed twice in Hanks' medium. The pellet, was resuspended in DMEM/F12 supplemented with B27, 25 mg/mL of bovine insulin, 100 mg/mL of transferrin, 20 nM progesterone, 100 µM putrescin, 30 nM sodium selenite, and 10 ng/mL of bFGF. The cell suspension was plated on culture plates (35-mm dishes) coated with 5% bovine serum albuminutes (BSA) at 3.0×105 cells/cm2 (3 mL of medium/dish). The effects of the following factors were evaluated, either alone or in combination: bFGF (10 ng/mL) and T3 (40 ng/mL). PSA-NCAM+ spheres that had been grown for five days on uncoated dishes in defined medium were prepared into single cells via trypsination and transferred to polyornithine/fibronectin-coated glass coverslips at low density for 3-5 days to determine the fate of cells derived from individual spheres. At that time, bFGF was omitted from the medium to allow differentiation. At days in vitro (DIV) 3 and DIV 7, spheres were also seeded onto coverslips in 100 µL droplets for 30 minutes, fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 minutes and rinsed with PBS to characterize the spheres (Fig. 1B).
Cells was stained with the following primary antibodies: monoclonal antibodies against PSA-NCAM (IgM, 1/200 dilution; Millipore, Billerica, MA, USA), nestin (IgG1, 1/50 dilution; BD INC., Franklin Lakes, NJ, USA), O4 (IgM, 1 : 500 dilution; R&D Systems, Minneapolis, MN, USA), GFAP (rabbit antibody, IgG, 1/500 dilution; Covance Research Products Princeton, NJ, USA), and βIII tubulin (IgG2a, 1 : 500 dilution; Dako Cytomation, Carpinteria, CA, USA). The following secondary antibodies were used at a 1/200 dilution (all obtained from Molecular Probes, Grand Island, NY, USA): Alexa Fluor 488 donkey anti-mouse IgG, Alexa Fluor 488 chicken anti-mouse IgG, Alexa Fluor 594 chicken anti-mouse IgG, Alexa Fluor 594 chicken anti-rabbit IgG, and tetramethylrhodamine isothiocyanate-conjugated goat anti mouse IgM. Both primary and secondary antibodies were diluted in 0.1% BSA containing 10% normal goat serum (NGS). Cells were saturated with 0.1% BSA containing 10% NGS and incubated with the primary antibody for one hour at room temperature. After rinsing with 0.1% BSA, the appropriate secondary antibodies were used, and then rinsed again with 0.1% BSA. Finally, they were rinsed and stained with Fluoromount-G including 0.1 µg/mL 4',6-diamidine-2'-phenylindole dihydrochloride (DAPI) (Vector Laboratories, Burlingame, CA, USA). The percentage of the various cell types was counted by the number of immunoreactive cells for each marker/the total number of DAPI+ cells.
The incorporation of 5-bromo-2-deoxyuridine (BrdU) (BD pharmingen, San Diego, CA, USA) was quantified by FACS for cell proliferation assay according to manufacturer' prosedure. In brief, the cells at DIV1, DIV2 and DIV3 were cultured for 16 hours in growth media containing 10 µM BrdU and then fixed in 5% glacial acetic acid, 95% ethanol for 5-10 minutes. Subsequently, the cells were incubated in 2N HCl for 10 minutes to denature DNA, and washed twice in 0.1N sodium borate (pH 8.5) for 10 minutes to neutralize the acid. After 3 washes with PBS, cells were incubated with anti-BrdU antibody (1 : 50 dilution), followed by incubation with a fluorescein conjugated goat anti-mouse IgG antibody (1 : 100 dilution) for 30 minutes, washed three times, counterstained with 1 mg/mL propidium iodide in 0.1% sodium citrate buffer for 10 minutes and visualized by confocal microscopy for cell counting.
When BrdU labeling was combined with surface labeling, cells were first fixed in 4% formaldehyde and incubated with PSA-NCAM antibody (dilution 1 : 250) for 45 minutes, followed by washing and incubation with rhodaminutese-conjugated anti-mouse IgM (dilution 1 : 100).6 The clusters were then post-fixed with acid-alcohol and processed for BrdU labeling as described above.
Total RNA of PSA-NCAM+ spheres at DIV5 was prepared with the RNeasy Kit (Qiagen, Valencia, CA, USA). For reverse transcription, 1 µg of RNA was transcribed into cDNA using Superscript II reverse transcriptase (Invitrogen, Grand Island, NY, USA) according to manufacturer' instructions. PCR reaction was performed with EmeraldAmp GT PCR Master Mix (Takara Bio Inc., Otsu, Shiga, Japan) using specific primers as previously reported.6
After the isolation process as described above, we were able to isolate a high purity of PSA-NACM+ cells (1-1.5×107 cells per 10 newborn rat brains). These cells, round morphologically, were identified by the nestin and PSA-NCAM markers. In response to bFGF, PSA-NCAM+ cells grew and aggregated gradually to form larger clusters on uncoated tissue culture dishes (Fig. 1C). Unfortunately, there is still no method of keeping PSA-NCAM+ cells for a long time. From the isolated PSA-NCAM+ cells, PSA disappeared completely in 7 days. In other words, these cells should be transplanted within six days (Fig. 2).
The combination of bFGF plus T3 produced a maximal increase in cell proliferation, whereas either one of the factors alone showed considerably low level of growth. To examine the effect of T3 on PSA-NCAM+ cell proliferation in response to bFGF, BrdU incorporation assay was performed between three and four days.
In the absence of T3 and bFGF, 2% of all cells were in DNA synthesis after three days in vitro. T3 alone did not significantly alter cell proliferation. The survival rate was 6.5-fold higher cells than in the untreated controls when only bFGF was added to the culture medium. In cultures treated with both bFGF and T3, however, survival rate was 9.5-fold higher than that of the untreated controls (p<0.01) and 1.5-fold higher than the cells treated with bFGF alone (p<0.05) (Fig. 3), suggesting that T3 could increase the proliferative response of PSA-NCAM+ cells only in the presence of bFGF (Fig. 3).
We next examined the differentiation potentials of PSA-NCAM+ cells after adhesion. All clusters/spheres were cultured under conditions with growth factors for five days and then transferred onto a dish or coverslip coated with polyornithine in the same medium without growth factors. Within a few hours, the cells migrated out of the clusters/spheres and grew processes, often showing a bipolar shape. After five days, many differentiated cells in the outgrowth were stained with O4 (oligodendrocytes), GFAP (astrocytes), and Tuj1 (neurons) antibodies. The great majority of pre-progenitor clusters showed GFAP+ plus O4+ cells. We then tested the fate of PSA-NCAM+ cell spheres grown with FGF2 and T3. These cells were differentiated into astrocytes (51%) and oligodendrocytes (44%), while only 5% neurons were observed. In the absence of T3 and FGF2 or with T3 only, only 20% oligodendrocytes were observed (p<0.01). In cultures treated with FGF2 only, 31% oligodendrocytes were observed (p<0.05) (Fig. 4).
Taken together, these results suggest that, T3 and bFGF may induce proliferation and promote an oligodendrocyte fate in these PSA-NCAM+ cells under our experimental conditions.
To determine whether our in vitro findings have physiological relevance, we studied the in vivo effects of T3 on ABCD2 expression in the PSA-NCAM+ cells, a major target of T3,8 and which can be transplanted for an X-ALD therapy. We observed induction of the gene in the PSA-NCAM+ cells, at the 40 ng/mL T3 and 10 ng/mL FGF2 dose, 1.8-fold higher than in untreated controls (p<0.05) and 1.7-fold higher than cells treated with FGF2 alone (p<0.05) (Fig. 5).
Herein, we determined whether the emergence of PSA-NCAM in vitro at the surface of neonatal rat brain precursors coincides with their restriction to a mostly glial fate. T3 modulates these events by enhancing PSA-NCAM+ cell growth in bFGF and favoring an OD fate. We also found the regulation of ABCD2 gene overexpression in PSA-NCAM+ cells, when they were treated with T3.
X-ALD is caused by mutations of the ABCD1, which encodes an ALD protein (ALDP), an ATP-binding cassette (ABC) transporter.1 ALDP function is required for entry of VLCFA, particularly C26:0 into peroxisomes where they are degraded by peroxisomal beta-oxidation.1 At abnormally high VLCFA levels, primary manifestations occur in the central nervous system (CNS), the adrenal cortex, and the testis's Leydig cells, however, the main pathology is the accumulation of cholesterol ester in the CNS, breakdown of myelin with relative sparing of axons, and a perivascular inflammatory response with breakdown of the blood-brain barrier.10 In boys and adolescents with early-stage cerebral involvement, bone marrow transplantation can provide long-term stabilization and occasionally even result in reversal of symptoms.11 The procedure, however, leads to clinical benefits only at an early stage of cerebral demyelination and remains associated with a high risk of mortality.12 Lorenzo's oil normalizes VLCFA levels in the plasma of X-ALD patients, but it does not alter the clinical progression in patients with neurological symptoms.13 Cell transplantation of myelin-producing exogenous cells is being extensively explored as a means of remyelinating CNS axons.3
In the context of remyelination strategies, there is a great deal of interest in finding ways to enhance the migration and differentiation of OD precursors.4,5 Cell adhesion molecules, such as NCAM, and its embryonic polysialylated isoforms, have also been implicated in the control of OD precursor migration, and the emergence of PSA-NCAM at the surface of neonatal brain precursors is found to coincide with their restriction to a glial fate.4 Here, T3 modulated these events by enhancing PSA-NCAM+ cell growth in the presence of bFGF and favoring an OD fate. Such distinct T3 effects might be mediated by different thyroid hormone receptors.14
There are three other closely related ABC half-transporters [ALDRP, PMP70 and PMP69 (PMP70R)] harboring peroxisomal membrane. These half-transporters are functionally associated and dimerization is required for full-function half-transport. Enforced expression of ALDRP can correct the biochemical defect in the ABCD1 knockout mouse.7 T3 induces ABCD2 overexpression in mature ODs from CG4 rat brains.7 The cerebral lesions in the rapidly progressive inflammatory forms of X-ALD resemble those of multiple sclerosis. Accumulation of cholesterol ester causes an inflammatory response.10 A study of the regulation of ABCD2 expression in PSA-NCAM+ cells would be of great interest because these cells can survive efficiently in X-ALD brain. In the present study, we confirmed that T3 also induces an overexpression of the ABCD2 gene in the PSA-NCAM+ cells.
X-ALD may be an example of a neurological disorder where new therapeutic strategies, such as gene modified cell therapy, will be required. PSA-NCAM+ cells, over-expressing ALDRP in high yield and high purity, are expected to extensively remyelinate CNS axons and efficiently survive in the X-ALD brain. Thus, the results described herein may provide new insights into investigating PSA-NCAM+ cells derived from human neural precursors for therapeutic application.
Figures and Tables
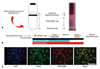 | Fig. 1(A) From neonatal Sprague Dawley rat brain, abundant PSA-NCAM+ cells were isolated by Percoll density gradient method. (B) The culture and differentiation protocol for PSA-NCAM+ cells. (C) Progenitor cells identified using the nestin and PSA-NCAM markers. Scale bar=50 µm. PSA, polysialylated; NCAM, neural cell adhesion molecule; DAPI, 4',6-diamidine-2'-phenylindole dihydrochloride; T3, 3,3',5-Triiodo-L-thyronine; bFGF, basic fibroblast growth factor. |
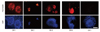 | Fig. 2In the PSA-NCAM+ cells, PSA disappeared in 7 days. Scale bar 50 µm. DIV, days in vitro. PSA, polysialylated; NCAM, neural cell adhesion molecule; DAPI, 4',6-diamidine-2'-phenylindole dihydrochloride. |
 | Fig. 3BrdU labeling shows that survival in cultures treated with both FGF2 and T3 was 9.5-fold higher than in the untreated controls (p=0.0075), T3 alone (p=0.0062) and 1.5-fold higher than cells treated with FGF2 alone (p=0.0433). *p<0.05; †p<0.01. BrdU, 5-bromo-2-deoxyuridine; T3, 3,3',5-Triiodo-L-thyronine; bFGF, basic fibroblast growth factor. |
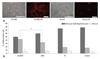 | Fig. 4(A) Oligodendrocytes identified using the O4 marker. (B) In PSA-NCAM+ cell, spheres grown with FGF2 and T3 differentiated into astrocytes (51%) and oligodendrocytes (44%), while only 5% neurons were observed. In the absence of T3 and FGF2 or with T3 only, only 20% oligodendrocytes were observed (p=0.0023), whereas 31% oligodendrocytes were observed in cultures treated with FGF2 only (p=0.035). Scale bar=50 µm. *p<0.05; †p<0.01. T3, 3,3',5-Triiodo-L-thyronine; bFGF, basic fibroblast growth factor; PSA, polysialylated; NCAM, neural cell adhesion molecule. |
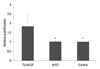 | Fig. 5The value of real-time quantitative polymerase chain reaction at the 40 ng/mL T3 and 10 ng/mL FGF2 dose. The induction of the ABCD2 gene in the PSA-NCAM+ cells was, 1.8-fold higher than in untreated controls (p=0.0325) and 1.7-fold higher than cells treated with FGF2 alone (p=0.0345). *p<0.05. T3, 3,3',5-Triiodo-L-thyronine; bFGF, basic fibroblast growth factor; PSA, polysialylated; NCAM, neural cell adhesion molecule. |
ACKNOWLEDGEMENTS
This work was supported by a grant (A100694) from the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea.
References
1. Moser HW. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain. 1997. 120(Pt 8):1485–1508.

2. Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009. 326:818–823.

3. Duncan ID. Oligodendrocytes and stem cell transplantation: their potential in the treatment of leukoencephalopathies. J Inherit Metab Dis. 2005. 28:357–368.

4. Decker L, Avellana-Adalid V, Nait-Oumesmar B, Durbec P, Baron-Van Evercooren A. Oligodendrocyte precursor migration and differentiation: combined effects of PSA residues, growth factors, and substrates. Mol Cell Neurosci. 2000. 16:422–439.

5. Grinspan J. Cells and signaling in oligodendrocyte development. J Neuropathol Exp Neurol. 2002. 61:297–306.

6. Ben-Hur T, Rogister B, Murray K, Rougon G, Dubois-Dalcq M. Growth and fate of PSA-NCAM+ precursors of the postnatal brain. J Neurosci. 1998. 18:5777–5788.

7. Kemp S, Wanders RJ. X-linked adrenoleukodystrophy: very long-chain fatty acid metabolism, ABC half-transporters and the complicated route to treatment. Mol Genet Metab. 2007. 90:268–276.

8. Fourcade S, Savary S, Gondcaille C, Berger J, Netik A, Cadepond F, et al. Thyroid hormone induction of the adrenoleukodystrophy-related gene (ABCD2). Mol Pharmacol. 2003. 63:1296–1303.

9. Lubetzki C, Goujet-Zalc C, Gansmüller A, Monge M, Brillat A, Zalc B. Morphological, biochemical, and functional characterization of bulk isolated glial progenitor cells. J Neurochem. 1991. 56:671–680.

10. Griffin DE, Moser HW, Mendoza Q, Moench TR, O'Toole S, Moser AB. Identification of the inflammatory cells in the central nervous system of patients with adrenoleukodystrophy. Ann Neurol. 1985. 18:660–664.

11. Moser HW, Tutschka PJ, Brown FR 3rd, Moser AE, Yeager AM, Singh I, et al. Bone marrow transplant in adrenoleukodystrophy. Neurology. 1984. 34:1410–1417.

12. Aubourg P, Blanche S, Jambaqué I, Rocchiccioli F, Kalifa G, Naud-Saudreau C, et al. Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med. 1990. 322:1860–1866.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download