Abstract
Purpose
We evaluated the effect of human parathyroid hormone (hPTH) on the engraftment and/or in vivo expansion of hematopoietic stem cells in an umbilical cord blood (UCB)-xenotransplantation model. In addition, we assessed its effect on the expression of cell adhesion molecules.
Materials and Methods
Female NOD/SCID mice received sublethal total body irradiation with a single dose of 250 cGy. Eighteen to 24 hours after irradiation, 1×107 human UCB-derived mononuclear cells (MNCs) and 5×106 human UCB-derived mesenchymal stem cells (MSCs) were infused via the tail vein. Mice were randomly divided into three groups: Group 1 mice received MNCs only, Group 2 received MNCs only and were then treated with hPTH, Group 3 mice received MNCs and MSCs, and were treated with hPTH.
Results
Engraftment was achieved in all the mice. Bone marrow cellularity was approximately 20% in Group 1, but 70-80% in the hPTH treated groups. Transplantation of MNCs together with MSCs had no additional effect on bone marrow cellularity. However, the proportion of human CD13 and CD33 myeloid progenitor cells was higher in Group 3, while the proportion of human CD34 did not differ significantly between the three groups. The proportion of CXCR4 cells in Group 3 was larger than in Groups 1 and 2 but without statistical significance.
Conclusion
We have demonstrated a positive effect of hPTH on stem cell proliferation and a possible synergistic effect of MSCs and hPTH on the proportion of human hematopoietic progenitor cells, in a xenotransplantation model. Clinical trials of the use of hPTH after stem cell transplantation should be considered.
Umbilical cord blood (UCB) is a useful alternative source of stem cells for patients without matched-related or matched-unrelated donors. However, the limited number of stem cells in UCB, and delayed engraftment, are major problems in UCB transplantation.1,2 To improve UCB engraftment, co-transplantation of mononuclear cells (MNCs) and mesenchymal stem cells (MSCs) has been attempted. MSCs are capable of supporting the expansion and differentiation of hematopoietic stem cells (HSCs) in vitro and enhancing hematopoietic engraftment in vivo, although the mechanisms are not fully understood.3
Other methods of improving engraftment include manipulation of homing and adhesion molecules, and modulation of the stem cell niche. The hematopoietic stem cell niche is a specialized microenvironment that contains stem cells, supports their maintenance, and regulates their function in bone marrow.4 Within bone marrow, HSCs that reside at, or near the endosteum as well as endosteal cells, secrete factors that promote HSC maintenance. Osteoblasts have been found to be important components of the niche. These produce factors that appear to regulate the maintenance and number of HSCs in bone marrow, including positive regulators such as angiopoietin,5 thrombopoietin and CXCL12,6,7 and negative regulators such as osteopontin.8,9 Bone also has a high concentration of calcium ions at the endosteal surface,10 and calcium-sensing receptors have been identified on the surfaces of HSCs. The ability of stem cells to sense and respond to the increased calcium concentration at the endosteal surface contributes to the unique stem cell-niche interaction that promotes bone marrow hematopoiesis.11
Osteoblasts produce hematopoietic growth factors and are activated by parathyroid hormone (PTH) or locally-produced parathyroid hormone-related protein (PTHrP) via the PTH/PTHrP receptor.12,13 PTH is recognized as one of the two major hormones modulating calcium and phosphate homeostasis. It is responsible for maintaining serum ionized calcium concentrations within a narrow range by stimulating renal tubular calcium reabsorption and bone resorption.14 Human parathyroid hormone (hPTH) 1-34 is an approved drug that has recently been used in large trials to treat osteoporosis, and was approved by the Food and Drug Administration for treatment of osteoporosis in postmenopausal women.15,16 Jung, et al.17 suggested that PTH increases stromal derived factor-1 expression in the bone marrow, which potentially contributes to homing, and Petrova, et al.18 demonstrated that PTH increases the expression of adhesion molecules for HSCs and might be used to promote the expansion of early HSCs ex vivo.
The purpose of the present investigation was to evaluate the effect of hPTH on the engraftment and/or in vivo expansion of HSCs in a UCB-xenotransplantation model. In addition, the role of hPTH in enhancing the expression of cell adhesion molecules was assessed.
The experimental protocol was reviewed and approved by the Institutional Review Board and the Institutional Animal Care and Use Committee of Hanyang University.
Six-week-old female NOD/SCID mice were purchased from the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea) and were maintained in sterile microisolator cages in a laminar airflow room in the Hanyang University Animal Laboratory. Mice were stabilized for 4 weeks and cared for according to the protocol described above.
All cells were provided by the Medipost Biomedical Research Institute (Seoul, Korea). UCB was collected from umbilical veins after neonatal delivery, with informed consent from pregnant mothers. MNCs were isolated from fresh UCB within 12 hours after collection. To isolate MNCs, UCB was fractionated using a Ficoll-Hypaque solution (d=1.077 g/cm3, Sigma-Aldrich, St. Louis, MO, USA) and MNCs were resuspended in PBS/EDTA and cultivated as previously reported.19,20 Briefly, the MNCs were maintained in α-minimum essential medium (α-MEM, Gibco BRL, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco BRL, Carlsbad, CA, USA), and seeded in culture flasks at 5×105 cells/cm2. Colonies of spindle-shaped cells were formed. At 50% confluence, the cells were harvested with 0.25% (w/v) trypsin-EDTA (Gibco BRL, Carlsbad, CA, USA) and reseeded for expansion. After the fourth passage, the cells were stored in liquid nitrogen at 1×106 cells/mL. The cells expressed CD105 (99.6%) and CD73 (96.3%), but not CD34 (0.1%), CD45 (0.2%) and CD14 (0.1%). They were positive for HLA-AB but generally not for HLA-DR. The human UCB-derived MSCs differentiated into various cell types such as osteoblasts, chondrocytes, and adipocytes upon in vitro induction with the appropriate osteogenic, chondrogenic, and adipogenic differentiation stimuli.
At ten weeks of age, all mice were weighed and received sublethal total body irradiation with a single dose of 250 cGy from a cesium source. Eighteen to 24 hours after irradiation, 1×107 human UCB-derived MNCs and 5×106 human UCB-derived MSCs were infused via the tail vein. The final volume infused was 200 µL. The mice were randomly divided into three groups: Group 1 (n=3) received MNCs only, Group 2 (n=3) received MNCs only and were then treated with hPTH, and Group 3 (n=3) received MNCs and MSCs, and were then treated with hPTH. 40 µg/kg/day of recombinant hPTH (Bachem, Torrance, CA, USA) was injected 20 times over the first 4 weeks after transplantation. The experimental schema is shown in Fig. 1.
Four and six weeks after transplantation, mice were anesthetized with diethyl ether (Sigma, Steinheim, Germany) and blood samples were collected via capillary tubes from their retroorbital venous plexus. An aliquot of the blood was used for complete blood cell counts. Seven weeks after transplantation, the mice were euthanized with xylazine hydrochloride (Rompun; Bayer HealthCare, Leverkusen, Germany) and zolazepam (Zoletil; Virbac SA, Carros, France). Blood samples were collected from the right ventricle after open thoracotomy. Complete blood count (CBC) was performed with an ABC Vet (Horiba ABX, Montpellier, France).
Both the femorae and tibiae were removed after eauthanasia. The proximal and distal ends of the femorae were cut and the marrow was aspirated into conical tubes with 2.0 mL of buffer (2% FBS, 2 mM EDTA, gentamicin). After centrifugation and red blood cell (RBC) lysis (BD Pharmingen, San Diago, CA, USA), the suspended cells were stained with anti-human CD13-PE, CD34-PE, CD45-FITC, CD33-PE, CD3-PE, CXCR4-PE, CD61-FITC, CD19-FITC (Becton-Dickinson, San Jose, CA, USA), CD41a-PE, CD49d-PE, and CD49e-PE (BD Pharmingen, San Diego, CA, USA) antibodies for 15 minutes at room temperature. The cells were washed with PBS and fixed with 1% paraformaldehyde (Sigma, Steinheim, Germany). They were analyzed with a FACSCaliber (BD, Los Angeles, CA, USA) and percentages of cell surface antigen expression were calculated among 5×105 events of the gated cells.
Excised tibiae were immersed in 10% paraformaldehyde for hematoxylin and eosin staining at 4℃, and then decalcified with 4% EDTA for 4 days at 4℃. Specimens were dehydrated with increasing concentrations of ethanol and embedded in paraffin. Paraffin-embedded tibiae were sliced into 5 µm sections and stained with hematoxylin and eosin to evaluate bone marrow cellularity.
CBC was within the normal range in the fourth week after transplantation in all three groups. At 4-, 6-, and 7- weeks after transplantation, there were no differences in WBC, RBC or platelet counts between the three groups (Fig. 2).
Bone marrow cellularity was approximately 20% in Group 1, but 70-80% in Groups 2 and 3. MSC co-transplantation had no effect on cellularity (Fig. 3).
Numbers of total nucleated cells (TNCs) in the bone marrow aspirates were 6.07±1.29×106 in Group 1, 10.53±2.25×106 in Group 2, and 8.33±3.25×106 in Group 3. There was a tendency toward higher TNCs in Groups 2 and 3 than in Group 1, but the differences were not statistically significant. The flow cytometric analysis confirmed engraftment of human UCB cells in all the mice. The representative flow cytometric images of 3 groups are shown in Fig. 4. The percentages of human CD34, CD33, CD13, CD19, and CD3 in the bone marrow aspirates are shown in Table 1. The proportions of myeloid lineage cells represented by human CD13 and human CD33 cells, and lymphoid lineage cells represented by human CD19 cells, were markedly higher in Group 3 (Fig. 5), but the proportions of human CD34 cells did not differ significantly between the three groups. In the analysis of human cell adhesion molecules on the nucleated cells in the bone marrow aspirates, the proportion of CXCR4 cells in Group 3 was larger than in Groups 1 and 2. However, the difference did not reach statistical significance (Fig. 6).
UCB has proved to be an alternative HSC source for patients without matched donors. Since the initial transplantation, more than 20000 UCB transplants have been performed worldwide.21,22 However, compared with bone marrow transplantation recipients and mobilized peripheral blood stem cell transplantation recipients, patients receiving UCB have prolonged recovery periods, which are reported to result from delayed neutrophil and platelet recovery.23 In addition, a graft failure rate of 10-15% has been reported. Possible causes include a low number of infused HSCs and the absence of a subpopulation that facilitates HSC engraftment.24 To overcome the low cell count in UCB and slow engraftment, several practical approaches have been attempted; for example, ex vivo expansion of UCB HSCs, transplantation of multiple units of UCB, co-transplantation of MSCs and other procedures.25-27 Ramírez, et al.28 have also shown that stem cell factor, interleukin (IL)-3, IL-6, and granulocyte macrophage colony-stimulating factor (GM-CSF) can induce an upregulation of several adhesion molecules
involved in the mobilization and homing of HSCs.
Bone marrow is composed with blood cells, non-blood cells and endosteum. Most hematopoietic progenitor cells are lined endosteum, whereas differentiated blood cells migrate to the medullar marrow cavity. Osteoblasts which are cells of the mesenchymal lineages contiguous to the bone surface execute the production of new bone and the control of osteoclast differentiation.29 Recently, Calvi, et al.13 demonstrated that overexpression of the hPTH/PTH-related peptide receptor under the control of the osteoblast specific α1(I) collagen promoter resulted in an increase of Sca-1- and c-kit-positive HSCs in bone marrow. An ability of hPTH to enhance transplant engraftment and repopulate bone marrow has also been reported.13 Only 27% of mice that were lethally irradiated and then injected with bone marrow cells from normal donor animals survived to 28 days. However, when the irradiated animals were injected daily with hPTH, all survived, with a larger number of transplanted marrow cells in their hind limbs. We also observed, in a previous study, that hPTH activates the insulin-like growth factor (IGF) system (IGF-2, IGF-BP 1, 2, 3), as well as hematopoietic growth factors (G-CSF, GM-CSF) from osteoblasts but not from MSCs (data not shown). In the present study, the proportion of human CD13 and human CD33 myeloid progenitor cells was much higher in Group 3, although the proportion of human CD34 did not differ significantly in the three groups. We also observed that the proportion of CXCR4 in Group 3 was larger than in Groups 1 and 2, though without statistical significance. These findings suggest that hPTH has the potential to enhance hematopoiesis as a result of activation of osteoblasts by co-transplanted MSCs.
Given the low number of HSCs in administered UCB, the outcome of HSC transplantation could be improved by co-transplantation of MSCs in addition to HSCs. MSCs have been shown to be able to express exogenous proteins for an extended period of time, and to maintain a higher stem cell proliferative capacity after transplantation in vitro.30 Briquet, et al.31 demonstrated that early passage MSCs supported the expansion of hematopoietic progenitor cells and their differentiation towards both B lymphoid and myeloid lineages.
In our study, an enhancing effect of hPTH on hematopoiesis was observed in a UCB xenotransplantation model with or without co-transplantation of MSCs. Although we could not demonstrate an effect of human PTH on differentiated osteoblasts from UCB-derived MSCs in an in vivo model, UCB transplantation together with co-transplantation of MSCs followed by administration of hPTH could be an option for enhancing hematopoietic activities and overcoming the current limitations of UCB transplantation. hPTH may enhance stem cell proliferation after treatment of chronic bone marrow aplasia by UCB transplantation and may also have a synergistic effect when MSCs are co-transplanted. Clinical trials with hPTH should be considered for patients with aplastic anemia as well as recipients of UCB.
Figures and Tables
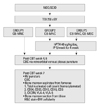 | Fig. 1Schema of the xenotransplantation experiment. TBI, total body irradiation; CB, cord blood; MNC, mononuclear cell; MSC, mesenchymal stem cell; hPTH, human parathyroid hormone; CBT, cord blood transplantation; CBC, complete blood count; RV, right ventricle; BM, bone marrow; IP, intraperitoneal; NOD/SCID. |
 | Fig. 2Mean complete blood cell counts of mice at the fourth, sixth, and seventh week after transplantation. There are no statistically significant differences between the 3 groups. RBC, red blood cell; WBC, white blood cell. |
 | Fig. 3Sections of the bone marrow of tibiae taken from mice of Groups 1, 2 and 3 in the eighth week after transplantation (H&E, ×200). The bone marrow cellularity was decreased to approximately 20% in group 1 (A). Bone marrow cellularity is approximately 70 to 80% in Groups 2 (B) and 3 (C). Bone marrow cellularity and trabecular bone formation are greater in Groups 2 and 3 than Group 1. |
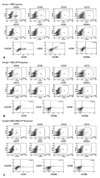 | Fig. 4The representative flow cytometric images of 3 groups. (A) Group 1, (B) Group 2, (C) Group 3. MNC, mononuclear cell; MSC, mesenchymal stem cell; hPTH, human parathyroid hormone. |
 | Fig. 5Flow cytometric analysis of engrafted human marrow mononuclear cells in bone marrow aspirates. (A) The percentage of myeloid lineage cells was larger in Group 3 than in Groups 1 and 2. (B) The percentage of lymphoid lineage cells was also higher in Group 3, but the difference was not statistically significant. |
ACKNOWLEDGEMENTS
This work was supported by a grant of Korea Healthcare Technology R&D Project (A101712), Ministry for Health & Welfare, Republic of Korea.
References
1. Kurtzberg J, Prasad VK, Carter SL, Wagner JE, Baxter-Lowe LA, Wall D, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008. 112:4318–4327.

2. Gluckman E, Rocha V, Arcese W, Michel G, Sanz G, Chan KW, et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004. 32:397–407.

3. Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005. 11:389–398.

4. Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008. 8:290–301.

5. Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004. 118:149–161.

6. Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007. 1:685–697.

7. Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002. 3:687–694.

8. Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grünewald E, et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005. 201:1781–1791.

9. Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005. 106:1232–1239.

10. Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006. 439:599–603.

11. Svinareva DA, Nifontova IN, Chertkov IL, Drize NI. Changed homing of hemopoietic precursor cells after long-term treatment with parathyroid hormone. Bull Exp Biol Med. 2006. 142:86–89.

12. Ballen K. Targeting the stem cell niche: squeezing blood from bones. Bone Marrow Transplant. 2007. 39:655–660.

13. Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003. 425:841–846.

14. Brown EM. Mechanisms underlying the regulation of parathyroid hormone secretion in vivo and in vitro. Curr Opin Nephrol Hypertens. 1993. 2:541–551.

15. Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003. 349:1216–1226.

16. Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003. 349:1207–1215.

17. Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006. 38:497–508.

18. Petrova NV, Svinareva DA, Nifontova IN, Momotyuk KS, Savchenko VG, Drize NI. Stromal regulation of hemopoietic stem cells in long-term human bone marrow tissue cultures under the effect of parathyroid hormone. Bull Exp Biol Med. 2006. 142:527–530.

19. Yang SE, Ha CW, Jung M, Jin HJ, Lee M, Song H, et al. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy. 2004. 6:476–486.

20. Jang YK, Jung DH, Jung MH, Kim DH, Yoo KH, Sung KW, et al. Mesenchymal stem cells feeder layer from human umbilical cord blood for ex vivo expanded growth and proliferation of hematopoietic progenitor cells. Ann Hematol. 2006. 85:212–225.

21. Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989. 321:1174–1178.

22. Gluckman E, Rocha V. Cord blood transplantation: state of the art. Haematologica. 2009. 94:451–454.

23. Szabolcs P. The immunobiology of cord blood transplantation. Korean J Hematol. 2010. 45:224–235.

24. Laughlin MJ. Umbilical cord blood for allogeneic transplantation in children and adults. Bone Marrow Transplant. 2001. 27:1–6.

25. Dorrell C, Gan OI, Pereira DS, Hawley RG, Dick JE. Expansion of human cord blood CD34(+)CD38(-) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood. 2000. 95:102–110.

26. Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001. 344:1870–1871.

27. Sauter C, Barker JN. Unrelated donor umbilical cord blood transplantation for the treatment of hematologic malignancies. Curr Opin Hematol. 2008. 15:568–575.

28. Ramírez M, Segovia JC, Benet I, Arbona C, Güenechea G, Blaya C, et al. Ex vivo expansion of umbilical cord blood (UCB) CD34 (+) cells alters the expression and function of alpha 4 beta 1 and alpha 5 beta 1 integrins. Br J Haematol. 2001. 115:213–221.

29. Garrett RW, Emerson SG. The role of parathyroid hormone and insulin-like growth factors in hematopoietic niches: physiology and pharmacology. Mol Cell Endocrinol. 2008. 288:6–10.

30. Kassem M. Mesenchymal stem cells: biological characteristics and potential clinical applications. Cloning Stem Cells. 2004. 6:369–374.

31. Briquet A, Dubois S, Bekaert S, Dolhet M, Beguin Y, Gothot A. Prolonged ex vivo culture of human bone marrow mesenchymal stem cells influences their supportive activity toward NOD/SCID-repopulating cells and committed progenitor cells of B lymphoid and myeloid lineages. Haematologica. 2010. 95:47–56.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download