Abstract
Purpose
Venous air embolism (VAE) is characterized by the entrainment of air or exogenous gases from broken venous vasculature into the central venous system. No study exists regarding the effect of patient positioning on the incidence of VAE during abdominal myomectomy. The purpose of this study was to assess the incidence and grade of VAE during abdominal myomectomy in the supine position in comparison to those in the head-up tilt position using transesophageal echocardiography.
Materials and Methods
In this study, 84 female patients of American Society of Anesthesiologist physical status I or II who were scheduled for myomectomy under general anesthesia were included. Patients were randomly divided into two groups: supine group and head-up tilt group. Transesophageal echocardiography images were videotaped throughout the surgery. The tapes were then reviewed for VAE grading.
Results
In the supine group, 10% of the patients showed no VAE. Moreover, 10% of the patients were classified as grade I VAE, while 50% were categorized as grade II, 22.5% as grade III, and 7.5% as grade IV. In the head-up tilt group, no VAE was detected in 43.2% of the patients. In addition, 18.2% of the patients were classified as grade I VAE, 31.8% as grade II, and 6.8% as grade III; no patients showed grade IV. VAE grade in the head-up tilt group was significantly lower than that in the supine group (p<0.001).
Venous air embolism (VAE) is characterized by the entrapment of air (or exogenous gases, such as carbon dioxide, nitrous oxide, nitrogen, and helium) from broken venous vasculature in the central venous system.1-7 VAE can induce systemic effects, such as thromboembolism, to the right heart or pulmonary artery.
The incidence of VAE has been shown to vary according to type of medical or surgical procedures.8 In posterior fossa surgery, the incidence of VAE was 76%.9 On the other hand, neurosurgical procedures in the other position show an incidence of 15% to 25%.10 Taken together, the position of patient is considered a major factor in the occurrence of VAE. The key pathophysiology of this phenomenon involves gravitational gradient. The height difference between broken venous vasculature and the right heart can create a negative pressure gradient.11 Moreover, a gravitational gradient as small as 5 cm has been reported to produce entrapment of large amounts of air that can result in emboli.10 In this sense, the degree of VAE may be influenced by the position of the patient.
During abdominal myomectomy, the uterus with fibroids is exteriorized for adequate exposure (Fig. 1). This procedure can result in the entrapment of air from broken uterine venous vasculature in the central venous system because of gravitational gradient.11,12 Although most cases of VAE are subclinical, VAE involves potentially life-threatening risks.13 Therefore, efforts to reduce the incidence of VAE are needed.
Several previous studies have reported on altering patient position to reduce the incidence of VAE. In patients undergoing cesarean section, results for the effect of a 5-10° head-up tilt position on the incidence of VAE are conflicting. One study reported that a 5° head-up tilt position reduced the incidence of VAE from 44% to 1%.14 However, other investigators reported that there was no significant difference in the incidence of VAE between the supine position and the 5-10° head-up tilt position.15 Although conflicting data exists for positioning during cesarean section, studies have yet to compare the effect of patient positioning on the incidence of VAE during abdominal myomectomy.
Therefore, the purpose of this study was to assess the incidence and grade of VAE during abdominal myomectomy in the supine position in comparison to those in the head-up tilt position using transesophageal echocardiography (TEE).
The study was approved by the Institutional Review Board, and written informed consent was obtained from all patients.
In this study, 84 female patients (20-55 years old) of American Society of Anesthesiologists physical status I or II who were scheduled for myomectomy under general anesthesia were included (Fig. 2). Patients with a history of prior abdominal surgery or cardiovascular or esophageal disease were excluded. Using a random number sequence, patients were randomly divided into two groups by a computer generator. Odd-numbered cases were allocated to the supine group (n=40), and even-numbered cases were allocated to the 10° head-up tilt group (n=44). All operations were performed by the same surgeon.
Patients were pre-medicated with midazolam (0.05 mg/kg, intramuscular injection) 60 minutes before induction of anesthesia. In the pre-anesthetic room, intravenous loading of lactated Ringer's solution was started. On arrival in the operating room, standard monitoring devices, including a three-lead electrocardiogram, non-invasive blood pressure, and pulse oximetry machines, were applied to the patient while in the supine position. All patients received 0.2 mg of glycopyrrolate intravenously. Anesthesia was induced with intravenous propofol (2 mg/kg) and remifentanil (1 µg/kg). Orotracheal intubation was then performed after intravenous administration of rocuronium (0.6 mg/kg). Anesthesia was maintained with sevoflurane (1.5-3 vol%) and remifentanil infusions (0.1 µg/kg/min). Neuromuscular relaxation was maintained with continuous intravenous infusion of rocuronium (2-5 µg/kg/min).
The patients' lungs were ventilated with 50% O2/50% air (2 L), maintaining an end-tidal CO2 partial pressure (PETCO2) of 35-40 mm Hg. Patients received approximately 1 L of lactated Ringer's solution just prior to skin incision. Afterwards, intraoperative fluid was subsequently administered with an infusion set (Control-A-Flo Set™ Model FMC5905, Baxter Ltd., Marsa, Malta) at a rate of 50 mL/hr to avoid echocardiographic artifacts caused by rapid intravenous infusion.16 After intubation, a 5.0-MHz multiplane TEE probe (SONOS 4500, Philips, Boeblingen, Germany) was inserted. Just after externalization of the uterus for myomectomy, patients in the head-up tilt group were tilted upward by 10°, while position in the supine group was unchanged.
The ultrasound gain setting was lowered to minimize artifact misinterpretation and three TEE views were recorded rapidly for later interpretation of the stage. The midesophageal (ME) 4-chamber view was continuously monitored during surgery and videotaped. When a bubble was detected in the right atrium (RA), the probe was turned to the right side and the angle was adjusted to the ME bicaval view to confirm its entrance from the inferior vena cava. Then, the angle was rapidly re-adjusted to view the ME right ventricle (RV) inflow-outflow view to confirm the extent of air embolism through the right ventricular outflow tract (RVOT). When gas bubbles filled more than half the diameter of the RA, RV, and RVOT, the ME 4-chamber, ME 2-chamber, ME long-axis, and transgastric mid short-axis views were obtained for visual assessment of left ventricular function and RV overload. No quantitative evaluation was performed. TEE images were videotaped throughout surgery.17 After surgery, two cardiac anesthesiologists blinded to patient group assignment reviewed the video tapes and graded VAE: grade I, single gas bubble in the RA, RV, and RVOT; grade II, gas bubbles filling less than half the diameter of the RA, RV, and RVOT; grade III, gas bubbles filling more than half the diameter of the RA, RV, and RVOT; and grade IV, gas bubbles completely filling the diameter of the RA, RV, and RVOT.18
Blood pressure (systolic, diastolic and mean), SpO2, and PETCO2 were recorded. Cardiovascular instability was defined as a sudden decrease of more than 20 mm Hg in the mean arterial blood pressure from the measurement taken 5 minutes prior, an acute fall of pulse oximetric saturation below 90%, and/or a sudden decrease of PETCO2 above 2 mm Hg from the baseline value. EKG was continuously monitored for changes related to VAE such as ST elevation, ST depression, paroxysmal supraventricular tachycardia, etc.
Sample size was predetermined using a power analysis based on the assumptions that 1) the incidence of VAE (above grade II) in patients undergoing myomectomy in the supine position would be about 70% (based on preliminary results), 2) the incidence of VAE (above grade II) in patients undergoing myomectomy in 10° head-up tilt would be decreased to about 40%, and 3) α=0.05 with a power (1-β) of 0.8. The analysis led us to conclude that 40 patients per group would be sufficient.
Statistical analyses were performed with SAS software (version 6.12, SAS Institute, Cary, NC, USA). The demographic and vital sign data between the two groups were compared using Student's t-test. The data of vital sign change during surgery in each group were compared using the repeated measures analysis of variance. Post-hoc analysis was done using the Bonferroni test. The grades of VAE between the two groups were compared using the chi-square test and Fisher's exact test. p-values <0.05 were considered statistically significant.
All 84 patients completed the protocol. The patient demographic characteristics were similar for both groups (Table 1). There were no specific differences in vital signs between the two groups, especially just after uterine exteriorization with or without position change (Table 2).
In the supine group, only 10% (4/40) of the patients showed no VAE. Moreover, 10% (4/40) of the patients showed grade I VAE, while 50% (20/40) showed grade II, 22.5% (9/40) grade III, and 7.5% (3/40) showed grade IV VAE on TEE.
In the head-up tilt group, VAE was absent in 43.2% (19/44) of the patients. In addition, 18.2% (8/44) of the patients showed grade I VAE, 31.8% (14/44) grade II, 6.8% (3/44) grade III, and nobody showed grade IV VAE (Table 3) (Fig. 3). The grade of VAE in the head-up tilt group was significantly lower than that in the supine group (p<0.001).
In 4 patients in the supine group and 2 patients in the head-up tilt group, a sudden decrease in PETCO2 above 2 mm Hg from the baseline value was noted. However, no other cardiovascular instabilities or EKG changes were encountered in either group. All VAEs in both groups occurred during excision of myoma from the exposed uterus.
In addition to abdominal myomectomy, new treatment methods have become available to patients with symptomatic fibroids including medical therapy, minimally invasive therapies such as uterine artery embolization or magnetic reson-ance-guided focused ultrasound surgery, and laparoscopic or vaginal myomectomy.19-22 Among these methods, abdominal myomectomy plays is invaluable because it is not limited to the size and number of fibroids that can be treated.23 However, abdominal myomectomy also involves the risk of VAE because the extent of uterine exteriorization necessary to achieve adequate exposure can produce greater gravitational gradient due to broken venous vasculature. Therefore, for this study, it was theorized that the grade and incidence of VAE during abdominal myomectomy could be reduced by reducing the gravitational gradient through patient positioning. In this study, the grade of VAE in the head-up tilt group was significantly lower than that in the supine group.
When VAE occurs in awake patients, the clinical manifestations include acute dyspnea, cough, and chest pain.24,25 Also, physical examination demonstrates rales, wheezing, and tachypnea. However, confirmation of the aforementioned symptoms and signs is not possible in anesthetized patients. Respiratory functions are therefore continuously monitored in patients under anesthesia. These monitors can detect decreases in PETCO2 and SpO2 along with hypercapnia; however, the low sensitivity and specificity of these monitors make it difficult to detect VAE.26 In this study, 4 patients from the supine group and 2 patients from the head-up tilt group showed a sudden decrease in PETCO2 >2 mm Hg from the baseline value.
TEE, used in this study, is currently the most sensitive monitoring method for VAE. TEE can detect air volumes as small as 0.02 mL/kg by bolus injection.27 However, TEE is relatively invasive and is not always obtainable in actual practice.
VAE can also provoke cardiovascular complications by right ventricular outflow obstruction. This phenomenon leads to hypotension and right heart failure. EKG changes generally present as a compromised cardiac status. EKG changes include ST-T changes that are followed by supraventricular and ventricular tachyarrhythmias.28 In this study, even a high VAE grade was not associated with cardiovascular instability, and there were no EKG changes observed for either group. These results are similar to those of other studies on the detection of VAE by TEE.16,17,29 However, clinicians should be aware of the risk of right-to-left shunt through which a direct cerebral air embolism is possible.
To minimize differences that might arise from having different surgeons perform the surgeries, in this study, all surgical procedures were performed by the same surgeon. Despite these efforts, the surgical complexity might have affected the incidence and stage of VAE. For example, more dissection in a situation of increased surgical complexity could have resulted in a higher degree of air exposure. In this study, patients with a history of prior abdominal surgery were excluded.
To avoid echocardiographic artifacts, we administered intra-operative fluid at a constant rate of 50 mL/hr, based on the study by Derouin, et al.16 We ascertained that no echocardiographic artifacts were seen on TEE at this infusion rate.
In conclusion, the grade of VAE in the head-up tilt group was significantly lower than that in the supine group without fluctuation of vital signs. Therefore, we suggest that the head up-tilt position during trans-abdominal myomectomy would be better in reducing VAE than the supine position.
Figures and Tables
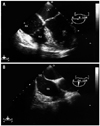 | Fig. 3Venous air emboli detected by transesophageal echocardiography during myomectomy. (A) Mid-esophageal four-chamber view. (B) Bicaval view. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle; IVC, inferior vena cava. |
Table 2
Changes in Vital Signs

SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; PR, pulse rate per minute; PETCO2, end-tidal CO2 partial pressure; PaO2, pulse oximetric saturation; TEE, transesophageal echocardiography.
The values are reported as mean±SD. There was no significant difference between the two group (p>0.05).
*The supine position was maintained and the values were checked just after uterine exteriorization.
References
1. Mommerot A, Perrault LP. Carbon dioxide embolism induced by endoscopic saphenous vein harvesting during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2006. 132:1502.

2. Park EY, Kwon JY, Kim KJ. Carbon dioxide embolism during laparoscopic surgery. Yonsei Med J. 2012. 53:459–466.

3. Kama NA. Influence of nitrous oxide anesthesia on venous gas embolism with carbon dioxide and helium during pneumoperitoneum. Surg Endosc. 2001. 15:1237–1238.

4. Cottin V, Viale JP, Bouffard Y, Delafosse B. Severe nitrous oxide embolism during venous stripping. Intensive Care Med. 1997. 23:1287–1288.

5. Boussuges A, Blanc F, Carturan D. Hemodynamic changes induced by recreational scuba diving. Chest. 2006. 129:1337–1343.

6. Risberg J, Englund M, Aanderud L, Eftedal O, Flook V, Thorsen E. Venous gas embolism in chamber attendants after hyperbaric exposure. Undersea Hyperb Med. 2004. 31:417–429.
7. Mitchell SJ, Benson M, Vadlamudi L, Miller P. Cerebral arterial gas embolism by helium: an unusual case successfully treated with hyperbaric oxygen and lidocaine. Ann Emerg Med. 2000. 35:300–303.

8. Kim CS, Liu J, Kwon JY, Shin SK, Kim KJ. Venous air embolism during surgery, especially cesarean delivery. J Korean Med Sci. 2008. 23:753–761.

9. Papadopoulos G, Kuhly P, Brock M, Rudolph KH, Link J, Eyrich K. Venous and paradoxical air embolism in the sitting position. A prospective study with transoesophageal echocardiography. Acta Neurochir (Wien). 1994. 126:140–143.

10. Albin MS, Carroll RG, Maroon JC. Clinical considerations concerning detection of venous air embolism. Neurosurgery. 1978. 3:380–384.

11. Maroon JC, Goodman JM, Horner TG, Campbell RL. Detection of minute venous air emboli with ultrasound. Surg Gynecol Obstet. 1968. 127:1236–1238.
12. Lang S. Precordial Doppler diagnosis of haemodynamically compromising air embolism during caesarean section. Can J Anaesth. 1991. 38:255–256.

13. Vacanti CA, Lodhia KL. Fatal massive air embolism during transurethral resection of the prostate. Anesthesiology. 1991. 74:186–187.

14. Fong J, Gadalla F, Druzin M. Venous emboli occurring caesarean section: the effect of patient position. Can J Anaesth. 1991. 38:191–195.

15. Karuparthy VR, Downing JW, Husain FJ, Knape KG, Blanchard J, Solomon D, et al. Incidence of venous air embolism during cesarean section is unchanged by the use of a 5 to 10 degree head-up tilt. Anesth Analg. 1989. 69:620–623.
16. Derouin M, Couture P, Boudreault D, Girard D, Gravel D. Detection of gas embolism by transesophageal echocardiography during laparoscopic cholecystectomy. Anesth Analg. 1996. 82:119–124.

17. Kim CS, Kim JY, Kwon JY, Choi SH, Na S, An J, et al. Venous air embolism during total laparoscopic hysterectomy: comparison to total abdominal hysterectomy. Anesthesiology. 2009. 111:50–54.
18. Schmandra TC, Mierdl S, Bauer H, Gutt C, Hanisch E. Transoesophageal echocardiography shows high risk of gas embolism during laparoscopic hepatic resection under carbon dioxide pneumoperitoneum. Br J Surg. 2002. 89:870–876.

19. Seinera P, Arisio R, Decko A, Farina C, Crana F. Laparoscopic myomectomy: indications, surgical technique and complications. Hum Reprod. 1997. 12:1927–1930.

20. Davies A, Hart R, Magos AL. The excision of uterine fibroids by vaginal myomectomy: a prospective study. Fertil Steril. 1999. 71:961–964.

21. Spies JB, Cooper JM, Worthington-Kirsch R, Lipman JC, Mills BB, Benenati JF. Outcome of uterine embolization and hysterectomy for leiomyomas: results of a multicenter study. Am J Obstet Gynecol. 2004. 191:22–31.

22. Hindley J, Gedroyc WM, Regan L, Stewart E, Tempany C, Hynyen K, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004. 183:1713–1719.

23. Gavai M, Berkes E, Lazar L, Fekete T, Takacs ZF, Urbancsek J, et al. Factors affecting reproductive outcome following abdominal myomectomy. J Assist Reprod Genet. 2007. 24:525–531.

24. Balki M, Manninen PH, McGuire GP, El-Beheiry H, Bernstein M. Venous air embolism during awake craniotomy in a supine patient. Can J Anaesth. 2003. 50:835–838.

25. Suarez S, Ornaque I, Fábregas N, Valero R, Carrero E. Venous air embolism during Parkinson surgery in patients with spontaneous ventilation. Anesth Analg. 1999. 88:793–794.

26. Souders JE. Pulmonary air embolism. J Clin Monit Comput. 2000. 16:375–383.
27. Furuya H, Suzuki T, Okumura F, Kishi Y, Uefuji T. Detection of air embolism by transesophageal echocardiography. Anesthesiology. 1983. 58:124–129.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download