Abstract
Purpose
To evaluate the outcome of transmesocolic (TMC) laparoscopic pyeloplasty compared with conventional laterocolic procedure for surgeons with limited experience.
Materials and Methods
We started laparoscopic pyeloplasty for ureteropelvic junction obstruction in 2009. Since then, 21 patients of left side disease have undergone this surgery in our institution. To access the left ureteropelvic junction, we used the conventional laterocolic approach in 9 patients, while the transmesocolic approach was used in the remaining 12 patients, and perioperative results and follow-up data were then compared.
Results
The mean operative time using the transmesocolic approach was significantly shorter than the conventional laterocolic approach (242 vs. 308 min, p=0.022). Furthermore, there was no complication or open conversion. Postoperative pain was significantly decreased in the TMC group (2.8 vs. 4.0 points, measured using the visual analogue scale on the first postoperative day, p=0.009). Postoperative complications were encountered in two patients. All patients were symptom-free after 1 year of follow-up, and radiologic success rates for each group were 92 and 89%, respectively.
Laparoscopic pyeloplasty (LP) has emerged as a feasible and reliable treatment option for ureteropelvic junction obstruction (UPJO), with a success rate equivalent to that of the classic open procedure.1-3 In addition, LP offers benefits associated with minimally invasive techniques, including less pain, shorter hospitalization, and better cosmesis.1,4,5
However, the use of LP is still limited because advanced laparoscopic skills are required.1,6 Several reports have discussed the stiff learning curve of LP.7-9 According to Inagaki, et al.,7 it generally takes over 5 hours for a surgeon to perform each of the initial 10 cases, and the time decreases to 3.5 hours with experience. In a study of 100 LP patients, Singh, et al.10 demonstrated the significance of learning curve with regard to operative time and complication rates.
Consequently, a variety of modifications have been suggested to ease technical difficulties associated with LP.1,7-9,11-13 One suggestion is to use the transmesocolic (TMC) approach. It is an alternative ureteropelvic junction (UPJ) approach that has been shown to reduce operative time compared to the standard laterocolic (LC) approach.14 It offers a direct path to the left UPJ through the mesocolon with less tissue dissection and bowel manipulation.14-17
The TMC approach may ease the learning curve of laparoscopic repair.16 However, a question remains whether this approach is superior to the LC approach even for surgeons who are not familiar with LP. Since we adopted LP in 2009, 26 patients with UPJO have undergone the procedure. From the beginning, we applied both TMC and conventional LC approaches. By analyzing those cases, we were able to compare both approaches in terms of associated learning curve.
Between March 2009 and February 2011, a total of 26 patients with UPJO underwent LP at our institution. Five patients had right UPJO and were excluded from this study. Among the remaining 21 patients, 18 of them were male (86%), and the mean age of all patients was 26.5 years (range: 2-76 years). The presence of hydronephrosis was detected by ultrasound or computed tomography, and the diagnosis of UPJO was confirmed by diuretic radionuclide renography (delayed urinary excretion: T1/2 >20 min). Two surgeons performed the whole series; each had prior laparoscopic experience (43 cases and 50 cases), but minimal experience with LP. The TMC apparoach was used in 12 patients. For the other 9 patients, the conventional LC pyeloplasty was performed. The decision to use TMC or LC approach was made intraoperatively (i.e. the mesocoloic field was inspected after achieving laparoscopic vision, and the TMC technique was selected if the mesocolon bulged enough to identify the renal pelvis lying behind).
Under general anesthesia, each patient was placed in a dorsal lithotomy position for the cystoscopy and retrograde pyelogram. After visualizing the ureter and narrowed UPJ, a 5-Fr, open-ended ureteral catheter (4-Fr for small children) was placed in the ureter with the tip positioned a few centimeters below the narrowed UPJ. The distal end of the catheter was wrapped in a sterile sheath.
Each patient was then placed in the lateral decubitus position. For the first trocar insertion, a Veress needle was inserted next to the umbilicus, and a pneumoperitoneum (12 mm Hg) was created. A 5-mm trocar was introduced paraumbilically. The peritoneal cavity was inspected using a 5-mm endoscope, and a 3-mm trocar was placed at the midclavicular line slightly caudal from the 5-mm port. Another 3-mm trocar was inserted at the lower third portion of the line between the xyphoid process and the umbilicus.
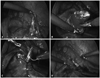
By rotating the patient laterally, the small bowel loops naturally fell down while the underlying colon remained fixed to the lateral abdominal wall. The dilated left renal pelvis and UPJ were visible behind the mesocolon, bulging to varying degrees. The anatomical position of the mesenterocolic space was defined cranially by the splenic flexure, laterally by the descending colon, medially by the gonadal vessels, and caudally by the left colic vessels (Fig. 1). In this area, the mesocolon was incised by laparoscopic scissors approximately 15 mm in length. The dilated pelvis was partially exposed and grasped. The UPJ and the proximal ureter were lifted, and surrounding tissues were dissected to fully expose the narrowed segment (Fig. 2A). Care was taken to preserve any accessory renal vessels supplying the lower renal pelvis and parenchyma. The lower renal pelvis was excised, and a holding stitch was placed on the partially-excised pelvis. The stitch and its thread were taken out through the lateral 3-mm port (Fig. 2B). This stitch worked as a renal pelvis traction tool and kept the renal pelvis out of the retroperitoneal space to stabilize it for completing the resection process and following sutures. It also prevented the operative field from sinking under the leaked urine and blood. After the narrowed UPJ portion was fully excised, it was displaced over the colon and the ureteral excision margin was spatulated for anastomosis.
The anastomosis suture was placed on the most dependent corner of the renal pelvis and the apex of the spatulated proximal ureter (Fig. 2C). Using a 5/0 monofilament interrupted suture, the posterior side was closed first. For the placement of a double-J ureteral stent, a guide wire was introduced through the open-ended ureteral catheter and grasped by atraumatic forceps. The guide wire tip was positioned inside the renal pelvis and the ureteral catheter was removed. Then, a 6-Fr double-J stent (4.7-Fr for small children) was inserted retrograde via the guide wire. The proximal J coil of the stent was positioned in the renal pelvis. The guide wire was removed. To prevent downward migration, the ureteral stent was grasped and held while the guide wire was pulled out through the urethra.
The remaining anterior wall anastomosis was completed using an interrupted 5/0 monofilament stitch (Fig. 2D). To verify the correct placement of the lower end of the stent, we performed cystoscopy in lithotomy position. The ureteral stent had a thread attached to its distal end, which aids in stent repositioning. If the stent's end was in the urethra, we pushed it inside the bladder by cystoscope. If it was misplaced in the ureter and only the thread was seen, we pulled the thread back in order to reposition the stent. A perianastomotic drain was usually not necessary. A urethral Foley catheter was left in the bladder for 1-2 days. The double-J ureteral stent was removed 4-6 weeks postoperatively.
After general anesthesia, a cystoscopy and retrograde pyelogram were performed. The patient was placed in a modified flank position. A transperitoneal three-port for a left-sided and four-port (additional 3-mm subxyphoid port for liver retraction) for a right-sided approach was used. After mobilization and medial displacement of the colon, the ureter was dissected superiorly until the UPJ was identified. The remainder of the procedure was same as that of the TMC laparoscopic procedure described above.
For postoperative pain control, Ketorolac (30 mg, IV, every 12 hours) was given for all adult patients on the first postoperative day. Tramadol (50 mg, IV) or Demerol (50 mg, IV or IM) was used later on patient's demand. For pediatric patients, ibuprofen syrup (5 mL for 2 year-old or younger, 8 mL for 3 to 6 year-old, 10 mL for older children) was given and ketorolac (0.5 mg/kg, IV) was also used when required. Pain was recorded using the visual analogue scale (VAS) every 8 hours by the assistance of the nursing staff. It was checked on the first postoperative day morning and rechecked again in the evening and night.
After institutional review board approval, surgical indication, operative time, complications, and outcomes were compared between TMC and LC patient groups. The operative time was measured from the start of cystoscopy to the closure of skin incision. Pain scale was estimated by using the mean value of VAS checked on the first postoperative day. Parameters to determine success were clinical resolution of symptoms and improved drainage on diuretic radionuclide scanning (T1/2 <20 min) performed 1 year post-operatively [The last patient of our series (12th TMC case) underwent radiologic study 6 months post-operatively].
Statistical data were analyzed using SPSS, version 18 (SPSS Inc., Chicago, IL, USA). Fisher's exact test was used for categorical variables and the Wilcoxon rank sum test for continuous variables. Statistical significance was set at p<0.05, and all reported p values were two-sided.
Patient characteristics are summarized in Table 1. TMC patients were generally younger than LC patients, but the difference was not statistically significant (20.4±19.2 years vs. 31.8±22.3 years, p=0.423). There were 4 pediatric patients (2 and 13 years old in TMC; 7 and 9 years old in LC). A dismembered Anderson-Hynes pyeloplasty was performed in all 21 patients. The TMC approach was used in 12 patients with left UPJO, and the LC approach was used in 9 patients. The other preoperative variables were not significantly different between the TMC and LC groups.
A significantly shorter operation time was achieved in the TMC group (242±63 vs. 308±76 min, p=0.022) (Table 2). There were 2 cases of redo-pyeloplasty. A 2-year-old boy underwent redo-pyeloplasty using the TMC approach, which took 270 min. A 28 year-old female patient also underwent redo-pyeloplasty using the conventional technique, which took 340 min. For a 53-year-old male patient who had an ipsilateral renal stone, intra-operative pyelolithotomy was performed with LC pyeloplasty.
No significant difference in intra-operative estimated blood loss was noted between the two groups (45±64 vs. 157±372 mL, p=0.082). Two patients had a history of ipsilateral percutaneous nephrostomy before the surgery. As the dilatation of the renal pelvis was not enough to bulge out the mesocolon, the LC approach was used for them. Two highest estimated blood losses, 1400 mL and 200 mL, were recorded in those 2 operations. Crossing vessels were observed in 4 patients (19%). All colic vessels were saved. No other complication or open conversion occurred during the procedures.
Postoperative pain was evaluated by the VAS scale on the first postoperative day, which was significantly lower in the TMC group (2.8±1.3 vs. 4.0±1.9, p=0.009). The mean hospital stay was similar for the TMC and LC groups (3.4±1.8 days vs. 3.6±1.7 days, p=0.923). During admission, one patient within the TMC group developed ileus. She was hospitalized for 8 days, which was the longest period of time among the patients in the TMC group. She recovered without further complications.
Mean follow-up durations were 12.1±5.9 months in the TMC and 12.4±5.9 months in the LC groups. The ureteral stent was removed 4 to 6 weeks after the surgery. During the follow-up, one LC patient developed a febrile urinary tract infection. He was admitted and treated with intravenous antibiotics. All patients reported symptomatic resolution or improvement. A radionuclide scan was performed 6 months to 1 year after the surgery, showing improved drainage in 11 of 12 TMC patients (91%) and 8 of 9 LC patients (89%).
Open pyeloplasty has been the standard treatment for UPJOs, but its associated significant morbidities have led to the development of minimally-invasive alternatives.18-20 LP has already become the preferred treatment option in many centers.2,11 Because of its less-invasive nature, several advantages (decreased postoperative pain, reduced hospital stay, and better cosmesis) have been observed.7,11 In addition, it can be used for both intrinsic and extrinsic causes of UPJO in a manner similar to the open pyeloplasty, which lacks of other minimally-invasive alternatives.21
However, there have also been reports to suggest technical difficulties and stiff learning curve of this complex reconstructive surgery1,9,13 Early reports indicate significantly longer operation time than that of the open procedure. For example, Moore, et al.22 reported in 1997 that the mean operative time of their initial 30 LP cases was 4.5 hours (range: 2.25 to 8 hours). In 2005, however, they recorded a mean operative time of 4 hours with 147 cases, which decreased with surgeon's experience.7
The technical difficulties of LP have been analyzed in several reports, and intracorporeal suturing was found to be the most-commonly noted time-consuming step, especially for beginners of LP. Alternative methods of anastomosis-such as fibrin glue and laser welding have been suggested, however, their long-term outcomes are not comparable.18-20
Recently, a direct transperitoneal access to the left UPJ has been proposed as an another shortcut for LP.14,15,17,23 Right sided pyeloplasties do not normally require extensive colonic mobilization. In contrast, the standard left side approach starts with a long vertical incision along the line of Toldt and subsequent dissection of the colonic flexure to move the colon medially and access the UPJ. This step generally consumes considerable time for beginners and creates surgical smoke and bleeding in the field, disrupting laparoscopic vision and consequently making the procedure difficult.14,16 The new TMC technique offers faster and safer access to the UPJ by avoiding colonic mobilization. Especially in cases where a redundant pelvis is present, mesocolic fat of the descending colon may be very thin or even transparent. With simple dissection of the thin layer, the underlying UPJ can be accessed. By avoiding bowel manipulation, this approach diminishes operative time, minimizes surgical smoke and bleeding, and consequently offers a clearer operative field.16
Romero, et al.14 reported good success rates by using both TMC and LC approaches. Specifically, the TMC approach offered a 22.5% reduction in operative time and a shorter hospital stay. The results are similar in subsequent other reports,15,17 even in surgeries involving children.24 We applied the TMC approach in 12 patients and also achieved a significantly shorter operative time than the conventional LC technique. As our surgeons were inexperienced for both approaches, the shorter operative time of the TMC group can be explained mainly by the simplicity of the TMC technique.
The TMC group indicated significantly less postoperative pain than the LC group. The reason for this finding could have several explanations. The manipulation of the colon and adjacent abdominal wall can cause visceral pain, nevertheless, it generally influences less the overall pain experienced after laparoscopic surgery.16 The longer operative time in the LC group may have caused more muscular pain (e.g., shoulder pain). Because there were no comparable data for analgesics used, the comparison may be of limited value. Castillo, et al.15 suggested a better convalescence for TMC pyeloplasty by avoiding colon mobilization, but they reported no difference in hospital stay or postoperative pain between the TMC and LC pyeloplasty patients.
Surgical outcomes of LP vary with the amount of experience, and mastering the procedure generally takes 20 to 50 consecutive cases.7,8,21,25 Our 21 left side LP operations were performed by two urologic surgeons with laparoscopic experience (43 and 50 cases) when they started LP. The numbers increased to approximately 100 cases of experience for both surgeons by the time when the 26th LP was performed. In the present study, the radigologic success rates were about 90% at the 1-year follow-up time point. And all preoperatively symptomatic patients (19 of 21 patients) were free of their initial symptoms. Although the cohorts were relatively small, the feasibility of the TMC approach was proven again, even for surgeons in their initial learning period of performing LPs. However, long-term follow-up is needed to confirm the advantages observed.
The TMC approach may encounter some problems. First of all, mesocolic fat can be thick and disturb the identification of the renal pelvis. Especially in older or obese patients, it may be thicker, which would cause troublesome bleeding during dissection. However, Porpiglia, et al.16 reviewed their 18 consecutive TMC LP cases and concluded that patient body habitus did not affect the outcome. They successfully performed TMC pyeloplasty in two patients with a body mass index of 28 kg/m2. In our study, we could easily identify protruding shape of the dilated renal pelvis in all TMC patients, regardless of their age and body habitus. We used the laterocolic approach in four patients with previous ureteral stenting or percutaneous nephrostomy. Because their renal pelvises were not dilated enough, TMC access would have been difficult. Braga, et al.26 suggested intraoperative renal pelvis dilation by injecting saline through an ureteral catheter. However, inserting an ureteral catheter preoperatively may interfere with the detection of a narrowed UPJ segment.27,28 Therefore, we used the LC approach in such cases.
The risk of losing grasp on the excised pelvis is an another possible problem. We made a stitch on the pelvis before fully excising the UPJ. The thread was taken out through the lateral trocar and held externally. The stitch prevented sudden cephalad migration of the renal pelvis, and also worked as a traction stabilizer during the anastomosis. Furthermore, leaked urine and blood from the opened renal pelvis made a blurring pool just under the operative field, and the pelvis was prevented from sinking under by pulling the renal pelvis upward with the holding stitch.
We performed cystoscopy and retrograde ureteropyelography before the laparoscopic procedure. Instead of inserting an ureteral stent into the renal pelvis, we initially placed a 5-Fr open-ended catheter with its tip placed just distal to the narrowed UPJ. Theoretically, preoperative ureteral stent placement may decompress the renal pelvis, render its dissection and mobilization more difficult, and may also impede intraoperative identification of the stenosis.27,28 Therefore, the UPJ should remain un-catheterized until it is accessed and the obstructed segment is excised. Then, the ureteral catheter can be moved upward and inserted to the renal pelvis. A guide wire can be then advanced retrograde through the catheter, and be later exchanged for a double-J ureteral stent.
In conclusion, the result of our study demonstrates that the TMC approach in LP had a comparable success rate to the LC approach in the initial period, with benefits of shorter operative time and less postoperative pain. This finding suggests that TMC pyeloplasty for left UPJO is better adopted by inexperienced surgeons than the LC approach. However, further experience is needed to verify the learning curves and long-term outcomes.
Figures and Tables
Fig. 2
(A) Transmesocolic approach. (B) Resection of pelvis and spatulation of ureter. (C) Anastomosis. (D) Anastomosis completed.
References
1. Moon DA, El-Shazly MA, Chang CM, Gianduzzo TR, Eden CG. Laparoscopic pyeloplasty: evolution of a new gold standard. Urology. 2006. 67:932–936.

2. El-Shazly MA, Moon DA, Eden CG. Laparoscopic pyeloplasty: status and review of literature. J Endourol. 2007. 21:673–678.

3. Mandhani A, Kumar D, Kumar A, Kapoor R, Dubey D, Srivastava A, et al. Safety profile and complications of transperitoneal laparoscopic pyeloplasty: a critical analysis. J Endourol. 2005. 19:797–802.

4. Sundaram CP, Grubb RL 3rd, Rehman J, Yan Y, Chen C, Landman J, et al. Laparoscopic pyeloplasty for secondary ureteropelvic junction obstruction. J Urol. 2003. 169:2037–2040.

5. Rassweiler JJ, Subotic S, Feist-Schwenk M, Sugiono M, Schulze M, Teber D, et al. Minimally invasive treatment of ureteropelvic junction obstruction: long-term experience with an algorithm for laser endopyelotomy and laparoscopic retroperitoneal pyeloplasty. J Urol. 2007. 177:1000–1005.

6. Shalhav AL, Orvieto MA, Chien GW, Mikhail AA, Zagaja GP, Zorn KC. Minimizing knot tying during reconstructive laparoscopic urology. Urology. 2006. 68:508–513.

7. Inagaki T, Rha KH, Ong AM, Kavoussi LR, Jarrett TW. Laparoscopic pyeloplasty: current status. BJU Int. 2005. 95:Suppl 2. 102–105.

8. Sorensen MD, Delostrinos C, Johnson MH, Grady RW, Lendvay TS. Comparison of the learning curve and outcomes of robotic assisted pediatric pyeloplasty. J Urol. 2011. 185:6 Suppl. 2517–2522.

9. Mandhani A, Kumar D, Kumar A, Dubey D, Kapoor R. Steps to reduce operative time in laparoscopic dismembered pyeloplasty for moderate to large renal pelvis. Urology. 2005. 66:981–984.

10. Singh O, Gupta SS, Arvind NK. Laparoscopic pyeloplasty: an analysis of first 100 cases and important lessons learned. Int Urol Nephrol. 2011. 43:85–90.

11. Türk IA, Davis JW, Winkelmann B, Deger S, Richter F, Fabrizio MD, et al. Laparoscopic dismembered pyeloplasty--the method of choice in the presence of an enlarged renal pelvis and crossing vessels. Eur Urol. 2002. 42:268–275.

12. Kaouk JH, Kuang W, Gill IS. Laparoscopic dismembered tubularized flap pyeloplasty: a novel technique. J Urol. 2002. 167:229–231.

13. Araki H, Ono Y, Hattori R, Goto M, Yamamoto T, Kimura T, et al. Laparoscopic pyeloplasty using endoscopic GIA stapler for ureteropelvic junction obstruction. J Endourol. 2005. 19:143–146.

14. Romero FR, Wagner AA, Trapp C, Permpongkosol S, Muntener M, Link RE, et al. Transmesenteric laparoscopic pyeloplasty. J Urol. 2006. 176(6 Pt 1):2526–2529.

15. Castillo OA, Vitagliano G, Alvarez JM, Pinto I, Toblli J. Transmesocolic pyeloplasty: experience of a single center. J Endourol. 2007. 21:415–418.

16. Porpiglia F, Billia M, Volpe A, Morra I, Scarpa RM. Transperitoneal left laparoscopic pyeloplasty with transmesocolic access to the pelvi-ureteric junction: technique description and results with a minimum follow-up of 1 year. BJU Int. 2008. 101:1024–1028.

17. Ramalingam M, Selvarajan K, Senthil K, Pai MG. Transmesocolic approach to laparoscopic pyeloplasty: our 8-year experience. J Laparoendosc Adv Surg Tech A. 2008. 18:194–198.

18. Eden CG, Sultana SR, Murray KH, Carruthers RK. Extraperitoneal laparoscopic dismembered fibrin-glued pyeloplasty: medium-term results. Br J Urol. 1997. 80:382–389.

19. Chiu AW, Lin CH, Huan SK, Liu CJ, Lin CC, Huang YL, et al. Creation of ureteropelvic junction obstruction and its correction by chemical glue-assisted laparoscopic dismembered pyeloplasty. J Endourol. 2003. 17:23–28.

20. Wolf JS Jr, Soble JJ, Nakada SY, Rayala HJ, Humphrey PA, Clayman RV, et al. Comparison of fibrin glue, laser weld, and mechanical suturing device for the laparoscopic closure of ureterotomy in a porcine model. J Urol. 1997. 157:1487–1492.

21. Ost MC, Kaye JD, Guttman MJ, Lee BR, Smith AD. Laparoscopic pyeloplasty versus antegrade endopyelotomy: comparison in 100 patients and a new algorithm for the minimally invasive treatment of ureteropelvic junction obstruction. Urology. 2005. 66:5 Suppl. 47–51.

22. Moore RG, Averch TD, Schulam PG, Adams JB 2nd, Chen RN, Kavoussi LR. Laparoscopic pyeloplasty: experience with the initial 30 cases. J Urol. 1997. 157:459–462.

23. Gupta NP, Mukherjee S, Nayyar R, Hemal AK, Kumar R. Transmesocolic robot-assisted pyeloplasty: single center experience. J Endourol. 2009. 23:945–948.

24. Sedláček J, Kočvara R, Molčan J, Dítě Z, Dvořáček J. Transmesocolic laparoscopic pyeloplasty in children: a standard approach for the left-side repair. J Pediatr Urol. 2010. 6:171–177.

26. Braga LH, Pippi-Salle J, Lorenzo AJ, Bagli D, Khoury AE, Farhat WA. Pediatric laparoscopic pyeloplasty in a referral center: lessons learned. J Endourol. 2007. 21:738–742.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download