Abstract
Purpose
The studies on the correlation between incidence of fall and brain atrophy have been going on to find out the cause of fall and its prevention. The purpose of this study was to explore the relationship between incidence of hip fracture and brain volume, measured by magnetic resonance image.
Materials and Methods
A total of 14 subjects with similar conditions (age, height, weight, and past history) were selected for this study. Fracture group (FG) was consisted of 5 subjects with intertrochanteric fracture. Control group (CG) had 9 subjects without intertrochanteric fracture. MRI-based brain volumetry was done in FG and CG with imaging software (V-works, CyberMed Co., Korea). Total brain (tBV), absolute cerebellar volumes (aCV) and relative cerebellar volumes (rCV) were compared between two groups. Student t-test was used to statistically analyze the results.
Results
In FG, average tBV, aCV and rCV were 1034.676±38.80, 108.648±76.80 and 10.50±0.72 cm3, respectively. In CG, average tBV, aCV and rCV were found to be 1106.459±89.15, 114.899±98.06 and 10.39±0.53 cm3, respectively, having no statistically significant difference (p>0.05).
Conclusion
There was no significant difference between the fracture and control groups. Patients with neurologic disease such as cerebellar ataxia definitely have high incidence of fall that causes fractures and have brain changes as well. However, FG without neurologic disease did not have brain volume change. We consider that high risk of fall with hip fracture might decrease brain function which is not obvious to pickup on MRI.
The annual worldwide incidence of hip fractures is estimated to be 1.6 million in 1990, and is expected to rise to 6.2 million by 2050.1 Hip fracture has become a major medical problem in recent years worldwide.2 The fracture seriously reduces a patient's quality of life and causes decline of life expectancy in about 20% of the fracture patients.3,4 The injury also propagates greater spending on caring and treating these patients.5 These hip fractures occur due to several factors; out of those, there is a direct correlation with the high incidence of fall.
The incidence of fracture resulting from a fall in the elderly is estimated to be around 1 to 5% of the fracture.6 More than one third of patients aged 65 years and above experience an episode of fall, and half of these may have repeated falls.7,8
After knowing the basic cause of fall and fall-related injury such as hip fracture, it seems possible to reduce and prevent such occurrence. Several authors had attempted to correlate the cause of fall in the group prone to fall with brain changes. In literature, degeneration of the frontal lobes, basal ganglion, or cerebellum as well as hydrocephalus have been proposed as possible brain lesions in groups prone to fall.9-11 Many researchers have been studying to find out the anatomical lesion related to faller without neurologic problems through imaging study such as computed tomography (CT) scan. Some authors have reported that there was little brain change in faller groups. However, there is no study regarding the relationship between the faller group with hip fracture and brain changes; even with the control group. The purpose of this study is to analyze the change of brain volume in hip fracture patients, who are prone to fall, using the magnetic resonance image (MRI) to calculate the 3D volume and to compare it with control group.
MRI was performed after informed consent in 14 subjects. All subjects have the same selection criteria (Table 1). Subjects were divided into two groups. For the first group, [fracture group (FG)] five patients were selected based on the fact that they fulfill the inclusion criteria and had sustained hip fracture within the last year. In the second group, [control group (CG)], nine subjects who matched the fracture group but did not have fracture were selected. Table 1 shows details of the inclusion criteria considering the factor affecting the brain size from the literature.
MRI was performed on 1.5-tesla Magneton vision (Simens, Erlangen, Germany). The following parameters were used for the volumetric acquisition: TR=9.7 msec, TE=4 msec, flip angle=12 degree, slice thickness=continuous 1.6 mm, matrix 192×256.
After the acquisition of MR images, the digital Imaging and Communications in Medicine files were transferred to an IBM compatible PC from Sun workstation with V-work software version 3.5 (CyberMed Co., Seoul, Korea).
Using 3D medical software package (CyberMed, Korea) used in a previous study,12 brain tissues on the MR images were separated from non-brain tissues (skull and meninges) for whole brain and cerebellar volumetric measurement. Threshold and region-growing technique was applied to eliminate any remaining meninges. The cut-off between the brainstem and spinal cord was the horizontal slice including cerebellum. Although this is somewhat arbitrary, there is no obvious and accepted gross anatomical landmark to distinguish brainstem from spinal cord on MR images. Using the last horizontal slice including cerebellum, in brains that were anterior commissure-posterior comminissure aligned, ensured that cut-off was reliable.13
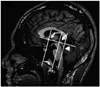
The cerebellum was segmented manually from the brainstem and cerebellar peduncles according to neuroanatomical landmarks13,14 and criteria similar to those adopted in the previous volumetric studies of the cerebellum.15,16 On sagittal slices, the cerebellar peduncles were removed from cerebellar white matter by following procedures shown in (Fig. 1): on the mid-sagittal slice a vertical line was drawn (at the angle of 90 degree to a line connecting anterior and posterior commissure) which touched posterior border of the inferior colliculus. This perpendicular line was manually traced as a part of the cerebellum (note in Fig. 1 that this included parts of the anterior lobe, biventer and flocculus on more lateral slices and of the tonsils and anterior lobe of the vermis on more medial slices). The final segmented cerebellum comprised the cerebellar hemispheres, deep nuclei and vermis.13
Two observers measured total brain volume (tBV), and absolute cerebellar volumes (aCV) and relative cerebellar volumes (rCV). The inter-observer reliability (two tailed, Pearson correlation coefficient) in tBV, aCV, and rCV was 0.94 (p=0.00), 0.93 (p=0.00) and 0.96 (p=0.00), respectively. The average of two observers was used for analysis.
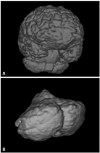
Values for tBV, aCV and rCV were calculated by summing the data which were obtained by multiplying each area slice thickness (1.6 mm), based on voxels (0.89×0.89×1.60 mm), using 3D model of the total brain and absolute cerebellum (Fig. 2). We normalized aCV to tBV in order to exclude inter-observer variability in tBV as a source of error in aCV measurement by calculating rCV in each subject as a percent ratio of their tBV, where rCV (%)=aCV/tBV×100. There is precedence in selecting brain volume or brain weight rather than body height to normalize brain morphometric data.13,17,18
Table 2 showed the results of brain volume (tBV, aCV, rCV) with p-value. The average tBV of FG was 1034.676±38.80 cm3, whereas that of CG was 1106.459±89.15 cm3. There was no significant difference between two groups (p>0.05). The tBV of both groups in this study was smaller than the results of Park, et al.19 Aging process might lead to brain atrophy.
The aCV of both groups was smaller than other results. The aCV was even smaller than that of average Korean (126±10.38 cm3) which was previously reported by Rhyu, et al.20 that cerebellar volume is not affected by aging. However, older subjects in this study seemed to have brain atrophy.
The average rCV of FG was 10.50±0.72, whereas that of CG was 10.39. There was also no significant difference between two groups, the results being almost the same.
Prevention of fall is an important issue to improve public health, especially in postmenopausal women. According to the literature, many protocols have been studied to decrease the rate of falls because of limitation in medical treatment which proved that 74% of hip fracture patients are non-osteoporotic.
In some cases, the antecedent of fall is readily identifiable, such as orthostatic hypotension, cardiac arrhythmias, or impaired gait from orthopedic or neurologic disability. In the majority of population, however, the causes of fall are not apparent. Despite the magnitude of the problem and the recent advances in brain imaging, few studies have addressed the potential contribution of CT or MRI identifying brain pathology that may be responsible for gait and balance impairment of the elderly people. A tendency for the group of gait-impaired elderly to have larger ventricles than the control group was found in three studies.21-23
Masdeu, et al.24 pointed out that the group of faller had a significantly greater degree of white matter hypodensity of the elderly. They have shown the relation between the faller and cerebral lesion. Hypodensity on CT appeared to be less parenchymal tissue in cerebrum, which indicated decline of brain volume. The above two groups used CT image, not MRI. In our present study, we measured a 3D MRI volume of brain which seems to be a realistic estimation. However, there was no significant difference between fracture and control groups. Koller, et al.22 also reported that only significant difference in supratentorial measures was dilatation of the lateral and third ventricle without changes in cerebral atrophy, in good agreement with our findings.
Degeneration of the cerebellar vermis results in gait disorder characterized by wide-based stance, inability to perform tandem walking, and truncal instability; characteristics similar to predominant signs observed in senile gait patients. However, there was no CT evidence suggestive of atrophy of the vermis or enlargement of the superior cerebella cistern.22 Paradiso, et al.25 demonstrated that the cerebellum may contribute to several aspect of cognition, showing that cerebellar volume was significantly correlated with the ability to retain already encoded information in the verbal domain and with fine motor dexterity. Cerebellar volume positively correlated in general; however the relationship did not show any statistical significance. The structural and functional relationships between cerebellum and verbal memory functions are consistent with evolutionary theory for the physiogenetical increase in the size of the cerebellum. Uncomplicated chronic alcoholism groups with ataxic deficit showed decline in cerebellar atrophy. They also mentioned the relation between the ataxia and cerebellar atrophy in normal population. Patients with cerebellar disease, such as cerebellar ataxia, definitely showed ataxic gait and increased risk of fall. However, the present study demonstrated no evidence that the fallers with hip fracture were related to the problem of balancing and postural instability, thus impliying that the cerebellar involvement is remote. Analyzing the above mentioned literature, each author advocated different and opposite results. In the literature, we found that most studies evaluated only CT scan, but not 3D MR images. To our best knowledge, there is no study to show direct relation between fall injury and brain volume. Therefore, even though this is a negative study, we think that this has an enough value as a preliminary study.
Although immense effort has been given to the control of subjects in parameters affecting the brain size (cerebral and cerebellum), especially body weight, height, intelligence and so on.26,27 In the present study, we did not address the factors affecting the postural instability, such as muscle strength. Significant associations have been found between muscle strength in the lower limb and falls,28 and a substantial proportion of elderly women have the risk of fall from muscle weakness while climbing steps, and reduced strength in plantar-flexor muscles considerably impairs the ability to generate stability torques at the ankle joint.29 The other factor to affect postural instability is peripheral sensory-motor function. Some authors have found greater levels of visual field dependence in elderly fallers compared with non-fallers.28
Our study has a few limitations. First, the number of subjects was too small because of the strict criteria for patient selection. Second, imaging study was done without clinical evaluation for the function of cerebellum. Most clinical evaluation of balance and postural stability is done on stance. Hip fracture patient cannot stand as they did before the injury. Thirdly, there are numerous variables that are found to affect the brain size. Therefore, further prospective study measuring relative volume (cerebral volume/intracranial volume) to calculate each part of cerebella volume would be mandatory to further explore this issue in detail.
Figures and Tables
Fig. 1
Landmark-based separation of the cerebellar peduncles from the cerebellar white matter and the brainstem. On the mid-sagittal slice (slice 46/90 in this subject), perpendicular lines are drawn at a 90 degree angle to bi-commissural line at the AC and PC (arrows) and through the posterior border of the inferior colliculus (arrowhead). These lines are superimposed on all sagittal slices and the latter perpendicular line is used to differentiate the cerebellar peduncles from the brainstem. AC, anterior commissure; PC, posterior comminissure.
References
1. Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997; 7:407–413.

2. Cummings SR, Kelsey JL, Nevitt MC, O'Dowd KJ. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985; 7:178–208.

3. Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr. 1999; 69:147–152.

4. Richmond J, Aharonoff GB, Zuckerman JD, Koval KJ. Mortality risk after hip fracture. 2003. J Orthop Trauma. 2003; 17:8 Suppl. S2–S5.
5. Sernbo I, Johnell O. Consequences of a hip fracture: a prospective study over 1 year. Osteoporos Int. 1993; 3:148–153.

6. Stevens JA, Olson S. Reducing falls and resulting hip fractures among older women. MMWR Recomm Rep. 2000; 49:3–12.

7. Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989; 261:2663–2668.

8. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988; 319:1701–1707.

9. Adams RD, Victor M, Ropper AH. Principles of Neurology. New York: McGraw-Hill;1977. p. 405.
10. Critchley M. On senile disorders of gait, including the so-called senile paraplegia. Geriatrics. 1948; 3:364–370.
11. Fisher CM. Hydrocephalus in walking problems of the elderly. Trans Am Neurol Assoc. 1980; 105:29–33.
12. Kim HJ, Yoon HR, Kim KD, Kang MK, Kwak HH, Park HD, et al. Personal-computer-based three-dimensional reconstruction and simulation of maxillary sinus. Surg Radiol Anat. 2003; 24:393–399.

13. Hutchinson S, Lee LH, Gaab N, Schlaug G. Cerebellar volume of musicians. Cereb Cortex. 2003; 13:943–949.

14. Press GA, Murakami J, Courchesne E, Berthoty DP, Grafe M, Wiley CA, et al. The cerebellum in sagittal plane--anatomic-MR correlation: 2. The cerebellar hemispheres. AJR Am J Roentgenol. 1989; 153:837–846.

15. Filipek PA, Richelme C, Kennedy DN, Caviness VS Jr. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994; 4:344–360.

16. Luft AR, Skalej M, Welte D, Kolb R, Bürk K, Schulz JB, et al. A new semiautomated, three-dimensional technique allowing precise quantification of total and regional cerebellar volume using MRI. Magn Reson Med. 1998; 40:143–151.

17. Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989; 112(Pt 3):799–835.

18. Peters M. Sex differences in human brain size and the general meaning of differences in brain size. Can J Psychol. 1991; 45:507–522.

19. Park IS, Han JW, Lee KJ, Lee NJ, Lee WT, Park KA, et al. Evaluation of morphological plasticity in the cerebella of basketball players with MRI. J Korean Med Sci. 2006; 21:342–346.

20. Rhyu IJ, Cho TH, Lee NJ, Uhm CS, Kim H, Suh YS. Magnetic resonance image-based cerebellar volumetry in healthy Korean adults. Neurosci Lett. 1999; 270:149–152.

21. Fisher CM. Hydrocephalus as a cause of disturbances of gait in the elderly. Neurology. 1982; 32:1358–1363.

22. Koller WC, Wilson RS, Glatt SL, Huckman MS, Fox JR. Senile gait: correlation with computed tomographic scans. Ann Neurol. 1983; 13:343–344.

23. Sudarsky L, Ronthal M. Gait disorders among elderly patients. A survey study of 50 patients. Arch Neurol. 1983; 40:740–743.
24. Masdeu JC, Lantos G, Wolfson L. Hemispheric white matter lesions in the elderly prone to falling. Acta Radiol Suppl. 1986; 369:392.
25. Paradiso S, Andreasen NC, O'Leary DS, Arndt S, Robinson RG. Cerebellar size and cognition: correlations with IQ, verbal memory and motor dexterity. Neuropsychiatry Neuropsychol Behav Neurol. 1997; 10:1–8.
26. Koh I, Lee MS, Lee NJ, Park KW, Kim KH, Kim H, et al. Body size effect on brain volume in Korean youth. Neuroreport. 2005; 16:2029–2032.

27. Spann W, Dustmann HO. [Weight of the human brain and its dependence on age, body length, cause of death and occupation]. Dtsch Z Gesamte Gerichtl Med. 1965; 56:299–317.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download