Abstract
Purpose
All structures of the spine, including the spinal canal, change continuously with age. The purpose of this study was to determine how the spinal canal of the lumbar spine changes with age. The L4/5 is the most common site of spinal stenosis and has the largest flexion-extension motion, whereas the T5/6 has the least motion. Therefore, we measured the spinal canal diameter and vertebral body height at T5, T6, L4, and L5 with age.
Materials and Methods
This was a retrospective study of aged 40 to 77 years. We reviewed whole spine sagittal MRIs of 370 patients with lumbar spinal stenosis (LSS) (Group 2) and 166 herniated cervical disc (HCD) (Group 1). Each group was divided into four age groups, and demographic parameters (age, gender, height, weight, BMI), the mid-spinal canal diameter, and mid-vertebrae height at T5, T6, L4, L5 were compared. Within- and between-group comparisons were made to evaluate changes by age and correlations were carried out to evaluate the relationships between all parameters.
Results
Height, weight, and all radiologic parameters were significantly lower in Group 2 than Group 1. Group 1 did not show any differences, when based on age, but in Group 2, height, weight, and T6, L4, and L5 height were significantly decreased in patients in their 70's than patients in their 40's, except for spinal canal diameter. Age was associated with all parameters except spinal canal diameter.
The spine is a flexible, multisegmented column that maintains stability and an upright position while providing mobility throughout life. The spine comprises static vertebral bodies and elastic joint complexes: the intervertebral disc and two posterior facet joints. Changes in the aging spine usually occur at movable intervertebral discs, facet joints, ligamentum flavum, and vertebral endplates which are adjacent to the intervertebral disc.1-4
Among the various problems with spinal aging, lumbar spinal stenosis (LSS) is the most frequent indication for spinal surgery in people over 60 years old.5 LSS refers to narrowing of the spinal canal, nerve root canals, or intervertebral foramina of the lumbar spine.6 Lumbar spinal stenosis can be congenital, degenerative, or both.5-9 Congenital stenosis is rare, while degenerative stenosis is more common, generally arises after the sixth decade of life, and is associated with age.5,6,10 Degenerative LSS is associated with degenerative changes of the elastic and moveable complex like intervertebral disc, zygapohyseal joints, and ligamentum flavum as dominant contributors.11-13 Recently, Abbas, et al.5 reported that the vertebral bodies and canals are significantly smaller in patients with degenerative LSS than in normal controls, and that both genetic factors (narrow spinal canal) and degenerative changes are associated with the pathogenesis of degenerative LSS. However, it is impossible to determine if the narrow spinal canal in degenerative LSS is degenerative change due to age or genetics. If the narrow spinal canal in degenerative LSS is associated with aging, degenerative LSS is degenerative disease. However, if the narrow spinal canal in degenerative LSS is not associated with aging, it appears to be logical to change the definition of degenerative LSS which reflects the genetic cause.
The purpose of this study was to determine how the mid-spinal canal diameter at L4 and L5 changes with age by using whole spine sagittal MRI. T5 and T6 have the least flexion-extension movement, while L4 and L5 have the largest flexion-extension movement, therefore, L4 and L5 are the most common sites for degenerative spine disease and degenerative spinal stenosis.14-17 Based on their movement, T5 and T6 are expected to show the smallest changes, and L4 and L5 to show the largest changes with age and degeneration, compared to the rest of the spine. Thus, to determine the changes in the spinal canal associated with age, we measured the diameter of the mid-spinal canal at T5, T6, L4, and L5. To clearly observe the change in the spinal canal diameter, we measured the bony mid-spinal canal diameter only, because the spinal canal at the disc space is influenced by changes in the intervertebral disc, facet joint, and vertebral endplate with age. In addition, patients with LSS experience strong degenerative changes with age, therefore, we included LSS in this study to observe a large variation. We compared LSS with herniated cervical disc (HCD) without thoracic and lumbar lesions as a control group.
This was a retrospective study of patients aged 40 to 77 years. The LSS group (n=370) included patients who underwent surgical treatment for L4-5 spinal stenosis between 2007 and 2011 (Group 2). All individuals in this group had intermittent claudication, often accompanied by other symptoms, such as radiculopathy, low back pain, or both. Before surgery, all patients underwent whole spine sagittal T2-weighted MRI with 1-mm cutting. We also included 166 patients who underwent surgical treatment for 1 segment HCD during same period as the controls (Group1). We excluded from this study any patients with multi-segment stenosis, disease at a vertebra other than L4 or L5, fractures, tumors, or patients who had a previous operation at any spinal segment. Any patients with abnormal findings of the thoracic or lumbar spine, except HCD, on a whole spine sagittal T2-weighted MRI were also excluded.
We compared demographic parameters (age, gender, height, weight, and BMI), and used PACS software and a PACS workstation (Centricity 2.0, GE Medical Systems, Milwaukee, WI, USA) to measure the anterior-posterior (AP) mid-spinal canal diameter and mid-vertebral body height. Using whole spine sagittal MRI, we selected the largest spinal canal image at the thoracic and lumbar spine. An independent neuroradiologist measured the anteroposterior diameter of the bony spinal canal on the mid-portion of the vertebral body, and mid-vertebral body height at T5, T6, L4, and L5 (Fig. 1). The patients were divided into four age groups: 40-49, 50-59, 60-69, and over 70.
Within- and between-group comparisons were made to-evaluate changes by age and correlations were carried out to evaluate the relationships between all parameters in all groups. SPSS for Windows version 15.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. Student t-tests were used to compare data between groups and between 40-49 year olds and over 70 year olds. Pearson's correlation was used to determine correlations with age. p-values <0.05 were considered statistically significant. No external funding were used for this study, and all procedures were approved by the Institutional Review Board of Gangnam Severance Hospital, Yonsei University College of Medicine (No 3-2012-0145).
The demographic characteristics of the two groups are summarized in Table 1. The 166 patients (110 males, 56 females) in Group 1 had mean age of 58.9±8.7 years, and the 370 patients (137 males, 233 females) in Group 2 had mean age of 62.2±9.0 years. Group 2 was shorter (165.4 vs. 160.4 cm, p=0.000) and weighed less (65.8 vs. 63.4 kg, p=0.008) than Group 1 (Table 1). When stratified by gender, there was no difference in height (male; 169.7 vs. 168.7, female; 156.9 vs. 155.5 cm) or weight (male; 70.1 vs. 70.8, female; 57.3 vs. 59.1 kg) between Groups 1 and 2.
In Group 1, there were no differences in height or weight based on age (Fig. 2, Table 1). In Group 2, over 70 year olds were shorter (165.4 vs. 160.4 cm, p=0.001) and weighed less (65.8 vs. 63.4 kg, p=0.035) than 40-49 year olds (Fig. 3, Table 1). When stratified by gender, there were no differences in Group 1. In Group 2, over 70 years old females were shorter than 40-49 years old (158.7 vs. 153.0 cm, p=0.001).
Table 2 summarizes the anterior-posterior (AP) spinal canal diameters and vertebral body heights for each group. All spinal canal diameters and vertebral body heights were significantly lower in Group 2 than Group 1 (Table 2). Among men, all spine canal diameters (T5; 14.8 vs. 14.3, p=0.001, T6; 15.0 vs. 14.6, p=0.017, L4; 16.9 vs. 15.2, p=0.000, L5; 17.7 vs. 15.7 mm, p=0.000) were smaller in Group 2. Among females, all spinal canal diameter (T5; 14.9 vs. 14.1, p=0.000, T6; 14.8 vs. 14.3, p=0.011, L4; 17.0 vs. 15.3, p=0.000, L5; 17.4 vs. 15.3 mm, p=0.000) and vertebral body heights at T5 (14.9 vs. 14.1 mm, p=0.000) and T6 (15.3 vs. 14.5 mm, p=0.000) were smaller in Group 2.
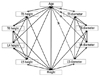
Within Group 1, there were no differences, when based on age (Fig. 4). In Group 2, the vertebral body height at T6 (15.9 vs. 15.0 mm, p=0.01), L4 (22.9 vs. 21.5 mm, p=0.000) and L5 (22.6 vs. 21.4 mm, p=0.002) was smaller in over 70 years old than 40-49 years old (Fig. 5, Table 2). Among males, T5 (16.2 vs. 15.2 mm, p=0.005) and L4 (23.4 vs. 21.1 mm, p=0.003) in Group 1 were shorter, and L4 was shorter (23.7 mm vs. 22.6 mm, p=0.043) in over 70 years old than 40-49 years old in Group 2. Females in both groups differed with age. Spinal canal diameter did not differ with age for all patients or when stratified by gender (Figs. 4 and 5, Table 2). Age was significantly correlated with all demographic characteristics and vertebral body heights, but did not correlate with spinal canal diameter (Fig. 6, Table 3).
Anatomically, LSS is caused by reduced space available for neural elements as a result of changes in the osseous and soft tissue elements surrounding the spinal column.5,11-13 Many previous studies have suggested that degenerative LSS begins with the loss of disc height resulting in annulus fibrosis and foraminal stenosis, and these changes alter the loading of the facet joints, leading to facet arthrosis, ligamentum flavum thickening, and osteophyte formation at the end-plate.11,18 These complex changes decrease the spinal canal diameter and dural sac dimension, which are related to aging.5 Because various changes due to aging may be related to pathologic spinal condition, like LSS, determining the changes in the aging spine is important.
However, anatomical studies of the aging spine are usually focused on intervertebral disc and facet joints, not the bony spinal canal, because these structures are elastic and moveable. While changes in the aging spine usually occur in these movable structures,1-4 bony lumbar vertebrae also change significantly, usually decreasing in height and broadening, and the vertebral body was posteriorly wedging at L4-L5 with aging.2,19-21 Twomey and Taylor4 reported that the bony lumbar spinal canal and foramina shrink with aging, and Abbas, et al.5 concluded that the vertebral bodies and bony spinal canals are significantly smaller in patients with degenerative LSS than in normal controls. However, Postacchini22 reported that pathologic narrowing of the nerve-root canal in LSS may not be associated with central spinal canal stenosis. Because these results are conflicting and do not indicate changes with age, it is not certain whether the smaller vertebral bodies and canals in degenerative LSS are due to genetic factors or age.
In the present study, we observed that spinal canal diameters in the LSS group were significantly lower than in the control group at both the largest movable (L4-5) and least movable segments (T5-6), similar to the study reported recently.5 However, this is the first study to indicate how the spinal canal diameter changes with age in LSS. We found that aging did not affect bony spinal canal diameters in either LSS or controls. Therefore, the narrow bony spinal canal in LSS is not due to aging, but rather genetics. Masharawi and Salame23 reported that age is not associated with spinal canal width and length variation in the thoracic and lumbar vertebrae in normal people.
We also observed that patients with LSS were shorter and weighed less than those with HCD. However, if we stratified the groups by gender, there was no difference. Only females with LSS were shorter with age. Although the bony spinal canal diameter was not affected by age, the mid-vertebral body height decreased in males with both HCD and LSS with age. As mentioned previously, aging and degenerative changes of the spine are focused at the intervertebral disc level, and mid-vertebral body height changes are associated with intervertebral disc changes.11,18 Shao, et al.1 reported that lumbar vertebrae became concave with age, and these changes mirror the convex changes in the disc and emphasize the importance of disc-vertebrae interactions with age. Mid-vertebral body height decreased in LSS with age, and is likely secondary to intervertebral disc aging.
This study has some limitations. First, we measured the diameter of the spinal canal from the whole spine sagittal images rather than axial cutting. To confirm the cross-sectional area of spinal canal, we need mid-vertebral body axial cutting MRI. Whole spine sagittal MRI was performed in patients with LSS and HCD before surgical treatment as a screening, therefore, we received only whole spine sagittal MRI and did not have thoracic axial cutting in LSS or lumbar and thoracic axial cutting in HCD. Further study should include whole spine axial cutting. Second, mean age (58.9 vs. 62.2 years), height (165.4 vs. 160.4 cm), and weight (65.8 vs. 63.4 kg) were different between groups. However, when stratified by gender, height and weight did not differ between groups or within each group based on age, except old female height with LSS. Despite these limitations, we found that, although the vertebral body height decreased with age, spinal canal diameter did not change with age in patients with either LSS or HCD. From this study, we think that the definition of "degenerative lumbar spinal stenosis" should be changed to reflect the genetic factor.
Figures and Tables
Fig. 1
Radiologic parameters of whole spine sagittal MRI. From the whole spine sagittal MRI, we selected the largest spinal canal image at the lumbar spine (A). The anteroposterior diameter of the spinal canal at the mid-portion of the vertebral body (white line) and mid-vertebra body height (white arrow) at L4 and L5 was measured (B). From the whole spine sagittal MRI, we selected the largest spinal canal image at the thoracic spine (C). The anteroposterior diameter of the spinal canal at the mid-portion of the vertebral body (white line) and mid-vertebra body height (white arrow) at T5 and T6 was measured (D).
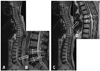
Fig. 3
Demographic characteristics in Group 2 (lumbar spinal stenosis). Comparisons between 40-49 year olds and over 70 year olds by Student's t-test, *p<0.01 and †p<0.05.

Fig. 5
Spinal canal diameters and vertebral body heights in Group 2 (lumbar spinal stenosis). Comparisons between 40-49 year olds and over 70 year olds by Student's t-test, *p<0.01.

Fig. 6
Correlations between all parameters. Age was significantly correlated with all demographic characteristics and vertebral body heights, but did not correlate with spinal canal diameter.
ACKNOWLEDGEMENTS
The authors thank Tae Sik Kim, at the Department of Neurosurgery, Gangnam Severance Hospital, for his effort in this study.
References
1. Shao Z, Rompe G, Schiltenwolf M. Radiographic changes in the lumbar intervertebral discs and lumbar vertebrae with age. Spine (Phila Pa 1976). 2002; 27:263–268.

2. Ericksen MF. Some aspects of aging in the lumbar spine. Am J Phys Anthropol. 1976; 45(3 pt. 2):575–580.

3. Vernon-Roberts B, Moore RJ, Fraser RD. The natural history of age-related disc degeneration: the influence of age and pathology on cell populations in the L4-L5 disc. Spine (Phila Pa 1976). 2008; 33:2767–2773.
4. Twomey L, Taylor J. Age changes in the lumbar spinal and intervertebral canals. Paraplegia. 1988; 26:238–249.

5. Abbas J, Hamoud K, May H, Hay O, Medlej B, Masharawi Y, et al. Degenerative lumbar spinal stenosis and lumbar spine configuration. Eur Spine J. 2010; 19:1865–1873.

6. Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res. 1976; 4–5.
7. Bartolozzi P, Salvi M, Misasi M. The diagnosis of lumbar stenosis. Chir Organi Mov. 1992; 77:15–18.
8. Eisenstein S. The morphometry and pathological anatomy of the lumbar spine in South African negroes and caucasoids with specific reference to spinal stenosis. J Bone Joint Surg Br. 1977; 59:173–180.

9. Katz JN, Dalgas M, Stucki G, Lipson SJ. Diagnosis of lumbar spinal stenosis. Rheum Dis Clin North Am. 1994; 20:471–483.

10. Singh K, Samartzis D, Vaccaro AR, Nassr A, Andersson GB, Yoon ST, et al. Congenital lumbar spinal stenosis: a prospective, control-matched, cohort radiographic analysis. Spine J. 2005; 5:615–622.

11. Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, Reilly J. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine (Phila Pa 1976). 1978; 3:319–328.

12. Kirkaldy-Willis WH, Paine KW, Cauchoix J, McIvor G. Lumbar spinal stenosis. Clin Orthop Relat Res. 1974; 30–50.
13. Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, Tchang S, de Korompay V, Shannon R. Lumbar spinal nerve lateral entrapment. Clin Orthop Relat Res. 1982; 171–178.

15. Panjabi MM, Goel V, Oxland T, Takata K, Duranceau J, Krag M, et al. Human lumbar vertebrae. Quantitative three-dimensional anatomy. Spine (Phila Pa 1976). 1992; 17:299–306.
16. Panjabi MM, Takata K, Goel V, Federico D, Oxland T, Duranceau J, et al. Thoracic human vertebrae. Quantitative three-dimensional anatomy. Spine (Phila Pa 1976). 1991; 16:888–901.

17. White AA 3rd, Panjabi MM. The basic kinematics of the human spine. A review of past and current knowledge. Spine (Phila Pa 1976). 1978; 3:12–20.
18. Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleâs F. Lumbar spinal stenosis: conservative or surgical management?: a prospective 10-year study. Spine (Phila Pa 1976). 2000; 25:1424–1435.
19. Ericksen MF. Aging in the lumber spine. III. L5. Am J Phys Anthropol. 1978; 48:247–250.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download