Abstract
Purpose
To investigate the ultrasonographic (US) features of anaplastic thyroid cancer (ATC) and the diagnostic performance of US-guided fine needle aspiration biopsy (FNAB) therein.
Materials and Methods
Eighteen cases of ATC diagnosed between January 2001 and May 2011 were included. FNAB was performed in all cases. Initial FNAB results were divided into three groups: 1) the cytological ATC group, cytological diagnosis of ATC; 2) the underestimated group, cytological diagnoses of malignancy other than ATC; and 3) the false negative group, cytological diagnoses of atypical, benign and non-diagnostic lesions. We retrospectively reviewed US findings and compared treatment modalities between each group.
Results
Among the 18 patients, there were nine in the initially cytological ATC group, four in the underestimated group and five in the false negative group. The most common US features of ATC were a solid (64.7%) and irregular shaped (88.2%) mass with lymph node involvement (76.4%). However, except for lymph node involvement (p=0.003), US findings for each group were not statistically different. The initial cytological diagnostic accuracy of ATC was 50% (9/18). Surgery was performed less in the ATC group (11%) and the false negative group (20%) than the underestimated group (75%).
Anaplastic thyroid cancer (ATC) is the most aggressive form of thyroid cancer, with median survival on the order of 3 to 5 months following diagnosis.1-4 Even though less than 1-3% of all thyroid cancers are ATC, it contributes to 14-50% of the annual mortality associated with thyroid cancer.5-7 The treatment for ATC has not been standardized because therapy has not been conclusively proven to be effective in prolonging survival. Until now, surgery has played an important role in survival for patients with intrathyroidal tumors.8-10 However, many patients have also presented with inoperable disease; macroscopic complete resection is only possible in up to one-third of patients at presentation.11-14 The best survival results have been observed in inoperable patients who received primary chemotherapy and radiation therapy rather than primary surgical resection.10,15 Therefore, an accurate preoperative diagnosis of ATC may help avoid unnecessary surgery and allow treatment to proceed directly to medical therapy.4,16
A diagnosis of ATC is usually suspected on clinical examination and ultrasonography (US), which can be confirmed by fine needle aspiration biopsy (FNAB), core biopsy or surgery. US and the following FNAB are the first diagnostic modalities in the evaluation of a palpable thyroid mass.17 Several studies have focused on the treatment modalities or outcomes of ATC,1,4,9,11,14,15,18,19 but there have been few reports about US features and the role of preoperative US-guided biopsy in ATC.
The purpose of this study was to investigate the US features of ATC and the diagnostic performance of US-guided FNAB.
This retrospective study was conducted with institutional review board approval and patient informed consent was waived.
From January 2001 to May 2011, a total 18 patients were diagnosed as having ATC by US-FNAB, core biopsy and surgery at our institution and were included in this study.
Initially, all patients had undergone US-FNAB of thyroid masses. According to the initial FNAB results, patients underwent follow up FNAB (n=2), core biopsy (n=3), diagnostic surgery (n=1) and other treatment options including medical treatment or therapeutic surgery (n=3). Among the 18 patients, 5 were men and 13 were women, with ages ranging from 54-81 years (mean age 70.5 years). All patients complained of having a palpable neck mass.
US was performed before FNAB in all 18 patients with a 7-15 MHz linear array transducer (HDI 3000 or 5000; Philips Medical Systems, Bothell, WA, USA) or 5-12 MHz linear array transducer (Iu22; Philips Medical Systems). Real-time US and subsequent US-FNAB was performed by one of six radiologists with 1 to 14 years of experience in thyroid imaging. US-FNAB was performed with a 23-gauge needle attached to a 20-mL disposable plastic syringe and aspirator or a 2-mL syringe disposable plastic syringe. Aspirated materials were expelled on glass slides and immediately fixed in 95% alcohol for Papanicolaou staining. The remaining material in the syringe was rinsed in normal saline for cell block processing. US-guided core biopsy was performed using a freehand technique, and each procedure was performed with a 18-gauge dual-action semiautomatic core biopsy needle (Stericut with coaxial; TSK Laboratory, Tochigi, Japan). A cytopathologist was not on site during the aspiration procedure. Additional special staining was performed according to the requirements set by the cytologist. From 2001 to December 2009, cytology reports were divided into the following categories: 1) nondiagnostic 2) benign 3) indeterminate 4) suspicious for malignancy and 5) malignant. Afterwards, the Bethesda classification was used to classify cytology results at our institution.20
Each US image was reviewed retrospectively by two radiologists in consensus. US images were reviewed according to size, composition, echogenicity, margin, presence of calcification, shape and presence of lymph node involvement. Lymph node involvement was considered pathologic if any one of the following suspicious features were present: round shape, increased echogenicity, cystic change, or presence of microcalcifications.21
To evaluate cytological diagnostic accuracy for ATC, FNAB results were divided into three groups: 1) the cytological ATC group, cytological diagnosis of ATC; 2) the underestimated group, cytological diagnoses of malignancy rather than ATC, such as suspicious for papillary carcinoma, papillary carcinoma, and poorly differentiated carcinoma; and 3) the false negative group, cytological diagnoses of atypical, benign and non-diagnostic lesions. We included atypia in the false negative group, because the Bethesda system recommends follow-up FNAB for patients with atypia instead of surgery.20 We compared US findings between these three groups.
For statistical analysis, the SPSS statistical package, version 11.0, for Windows (SPSS Inc., Chicago, IL, USA) was used. Pearson chi square tests and Fisher's exact tests were used to study categorical variables and the Kruskal-Wallis test was used to compare continuous variables. p-values < 0.005 were considered statistically significant.
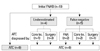
Among the 18 patients, there were nine in the initially cytological ATC group; four in the underestimated group, poorly differentiated cancer (n=3) and suspicious papillary carcinoma (n=1); and five in the false negative group, atypia (n=1), benign (n=3), and non-diagnostic (n=1). The initial cytological diagnostic accuracy of ATC was 50% (9/18) and it increased to 72% (13/18) if the underestimated group was regarded as part of the correct diagnostic group. Among the 4 patients in the underestimated group, one case of suspicious papillary carcinoma was diagnosed as ATC by follow-up core biopsy, and three cases of poorly differentiated thyroid carcinoma (PDTC) that underwent surgery were finally diagnosed as ATC. Among the 5 patients in the false negative group, one atypia case and one non-diagnostic case were diagnosed as ATC by follow-up core biopsy; two benign cases were diagnosed as ATC by repeat FNAB and one benign case was determined to be ATC after surgery (Fig. 1). Accordingly, a preoperative diagnosis of ATC was made in 14 out of 18 cases (77.8%). The mean number of days from first visit to confirmative diagnosis of ATC was 10 days for the ATC group, 33.5 days for the underestimate group and 104.5 days for the false negative group.
US images were available in 17 out of 18 patients. US features of the 17 ATC patients according to the three groups above are summarized in Table 1. Mean age and mass size were not statistically different between each group. The common US features of ATCs were solid (11/17, 64.7%), marked hypoechogenicity (9/17, 52.9%) irregular margin (15/17, 88.2%), internal calcification (9/17, 52.9%), wider than tall shape (12/17, 70.5%), and presence of cervical lymph node involvement (13/17, 76.4%). The US findings for each group were not statistically different, except for lymph node involvement (p=0.003). Mixed composition was more frequently seen in the underestimated (50%) and false negative groups (60%), compared with the ATC group (12.5%), but this was not statistically different (p=0.262). We also compared US findings between those in the initially diagnosed malignancy group (the cytological ATC and underestimated groups) and those in the false negative group, but similarly, lymph node involvement was the only statistically factor that differed between the two groups (Table 2).
Differences in treatment modalities between the three groups are summarized in Table 3. Surgery was performed less on patients in the initially confirmed ATC (11%) and false negative groups (20%) than those in the underestimated group (75%).
ATC is an extremely aggressive solid tumor that resists most therapeutic efforts and is always fatal.1-4,9,10 It contrasts with well differentiated thyroid cancer (WDTC), which accounts for most thyroid malignancies, with an indolent course and good prognosis regardless of the type of treatment.2 The incidence of ATC is decreasing, presumably due to earlier detection of antecedent disease.22 Several studies have revealed that potentially curative surgery is the only discriminating variable that retains a significant association with prolonged survival.8,9,18 But, it was seen in previous studies that systemic metastases were present in 46% of ATC patients at presentation, and 68% ATC of patients had metastases diagnosed during the course of their illness.19 At this stage, complete resection is not meaningful, so patients with inoperable disease at diagnosis are considered for combined chemotherapy and radiotherapy.10 Thus it is important to accurately diagnose ATC before surgery and treatment.16
Grossly, ATC is a nonencapsulated, tan-white, fleshy tumor with a direct extension into the surrounding soft tissue of the neck and has regions of necrosis and hemorrhage.23 The imaging features of ATC reflect these gross findings. Large, solid and ill defined masses accompanied by necrosis, nodular calcification and cervical lymph node involvement are the common image features of ATC.24 In our study, common US features of ATCs included solid (11/17, 64.7%), marked hypoechogenicity (9/17, 52.9%), irregular margin (15/17, 88.2%), internal calcification (9/17, 52.9%), wider than tall shape (12/17, 70.5%) and presence of cervical lymph node involvement (13/17, 76.4%). The image features of ATCs are not that different from other aggressive forms of thyroid cancer, but the prognosis of ATC is significantly worse than PDTC. The 5-year disease free survival and 5-year cause-specific survival of ATC are both 0%, but those of PDTC are 51% and 70%.25 PDTC patients survive longer, with medial survival being 3.2 years for PDTC and 3.1 months for ATC. In a previous study, one third of ATC patients died of local disease, whereas all patients who died of PDTC had distant metastases.26 ATC more frequently invades the surrounding structures and more often involves regional lymph nodes; moreover, half the patients with ATC present with distant metastases.23
Considering the limitations of US evaluation in studying larger masses, CT is much more useful in defining the local extent of the disease and detecting lymph node metastases. MRI has further added value over CT scan for detecting invasion of vascular, airway and bony structures.23,27 CT or MR information on the extent and location of tumor necrosis and site of calcification in the tumor is expected to lower false negative diagnoses by appreciating indication sites for biopsy.28 However, when ATC is clinically suspected, US-guided cytologic confirmation is initially performed and CT or MRI is performed after diagnosis.
The cytological diagnosis accuracy of ATC reported by previous reports was 78.7% to 90%.29-33 In the present study, the accuracy of initial US-FNAB was 50% (9/18) and increased to 72% (12/18) if the underestimated group was included. The diagnostic performance in our study was lower than others and this may be due to a different study design and sample size. Previously, Us-Krasovec, et al.29 reported the FNAB results of 113 ATCs for 20 years. They reported that 107 patients (107/113, 94.7%) were diagnosed as malignant and 96 (96/107, 89.7%) were diagnosed as ATC through reexamination. If we had reevaluated the pathology slides retrospectively perhaps there would have been an increase in diagnostic accuracy. The reason for failures in obtaining an accurate diagnosis on FNAB might have been due to sampling in areas that consisted of foci of WDTC or in areas burdened with necrosis, fibrosis, or hemorrhage.2,29 In the present study, among the five false negative cases, 60% (3/5) had both solid and cystic portions on US, which contrasted with the 12.5% (1/8) in the initially cytological ATC group. It is difficult to obtain adequate cells for diagnosis from masses containing both solid and cystic portions,34,35 which could explain why false negative diagnosis was frequent in mixed composition.29 According to Luze, et al.,31 12% of samples of finally confirmed ATCs contained only necrotic material. In order to obtain a representative sample, large tumor masses must be aspirated at two or three different sites. This is particularly important in differentiated tumors undergoing transformation to ATC, in which only small foci of ATC components may be present and both well differentiated carcinoma and ATC coexist.1,2,29
Multimodality treatment with combination surgery, radiation and chemotherapy has been reported to improve outcomes for ATC.2,3,5,18 If tumors can be managed by aggressive surgery with preservation of organ function, adjuvant chemotherapy and radiotherapy may be the best option for now.36 The majority of patients with ATCs die from aggressive local regional disease, primarily upper airway respiratory failure. For this reason, aggressive local therapy is indicated in all patients who can tolerate it. Yet complete resection of ATC is rarely performed, which gives the best chance of long term control and disease free survival. Accordingly, more than two-thirds of advanced ATC patients are not expected to show survival benefits from initial surgery.37 In our study, 92.8% (13/14) of pre-op diagnosed ATC cases did not undergo surgical treatment. Among the 9 patients in the cytological ATC group, only 1 patient underwent surgery, whereas 3 out of 4 patients in the underestimated group underwent surgery even though similar US findings were observed. If the patients had been diagnosed with ATCs preoperatively, surgery would not have been performed.
Among the 9 patients in the underestimate and false negative groups, 5 patients were diagnosed as having ATCs through repeat FNAB or core biopsy. Repeated FNAB and core biopsy including immunohistochemical staining and flow cytometry analysis2,19,22 were valuable methods when suspicions of clinical and imaging findings remained high and initial FNAB was inadequate or benign.
There are some limitations to this study. First, this study was based on a retrospective design, and the initial cytology results were not reviewed. More over as this study had a small sample size, we did not compare the mean survival period of each group by different treatment modalities. So we did not analyze the survival rate of each group. Second, almost all cases of ATC in our study were of large size and advanced cases. Recently, the wide use of high resolution US has provided early detection of thyroid malignancy, and this might contribute to an earlier detection of ATC. Han, et al.38 reported time trends of tumor size and characteristics of ATC. They reported that the mean tumor size of ATC decreased significantly and the frequencies of coexistent differentiated thyroid cancer (DTC) increased over time. The mean tumor size of long term survivors was significant smaller and the proportion of cases with coexistent DTC was much higher in long term survivors than short term survivors. So smaller ATC and cases with coexistent DTC were increasingly detected, and accordingly, therapeutic approaches have evolved with growing expectations of long term survival for ATC patients.38
In conclusion, in the present study, the most common US features of ATC were a large, solid, irregular shaped mass with lymph node involvement. A correct diagnosis of ATC by initial US-FNAB was made in 50% of the patients, which is significant in that therapeutic surgery can be undertaken in lower numbers if correctly diagnosed.
Figures and Tables
Fig. 1
Preoperative diagnostic methods of 18 anaplastic ATC. Underestimated group: poorly differentiated cancer and suspicious papillary carcinoma. False negative group: atypia, benign and non-diagnostic. bx, biopsy; ATC, anaplastic thyroid cancer; FNAB, fine needle aspiration biopsy.
References
1. Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006; 13:453–464.

2. Patel KN, Shaha AR. Poorly differentiated and anaplastic thyroid cancer. Cancer Control. 2006; 13:119–128.

3. Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol. 2011; 2011:542358.

5. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007; 57:43–66.

6. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer. 1998; 83:2638–2648.

7. Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005; 103:1330–1335.
8. Sugino K, Ito K, Mimura T, Nagahama M, Fukunari N, Kubo A, et al. The important role of operations in the management of anaplastic thyroid carcinoma. Surgery. 2002; 131:245–248.

9. Akaishi J, Sugino K, Kitagawa W, Nagahama M, Kameyama K, Shimizu K, et al. Prognostic factors and treatment outcomes of 100 cases of anaplastic thyroid carcinoma. Thyroid. 2011; 21:1183–1189.

10. Pudney D, Lau H, Ruether JD, Falck V. Clinical experience of the multimodality management of anaplastic thyroid cancer and literature review. Thyroid. 2007; 17:1243–1250.

11. Heron DE, Karimpour S, Grigsby PW. Anaplastic thyroid carcinoma: comparison of conventional radiotherapy and hyperfractionation chemoradiotherapy in two groups. Am J Clin Oncol. 2002; 25:442–446.
13. Mitchell G, Huddart R, Harmer C. Phase II evaluation of high dose accelerated radiotherapy for anaplastic thyroid carcinoma. Radiother Oncol. 1999; 50:33–38.

14. McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001; 130:1028–1034.

15. Besic N, Auersperg M, Us-Krasovec M, Golouh R, Frkovic-Grazio S, Vodnik A. Effect of primary treatment on survival in anaplastic thyroid carcinoma. Eur J Surg Oncol. 2001; 27:260–264.

16. Greenblatt DY, Woltman T, Harter J, Starling J, Mack E, Chen H. Fine-needle aspiration optimizes surgical management in patients with thyroid cancer. Ann Surg Oncol. 2006; 13:859–863.

17. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.

18. Haigh PI, Ituarte PH, Wu HS, Treseler PA, Posner MD, Quivey JM, et al. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001; 91:2335–2342.

19. Lam KY, Lo CY, Chan KW, Wan KY. Insular and anaplastic carcinoma of the thyroid: a 45-year comparative study at a single institution and a review of the significance of p53 and p21. Ann Surg. 2000; 231:329–338.
20. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009; 19:1159–1165.

21. Rashid OM, Takabe K. The evolution of the role of surgery in the management of breast cancer lung metastasis. J Thorac Dis. 2012; 4:420–424.
22. Boerner SL, Asa SL. Biopsy interpretation of the thyroid. 1st ed. Philadelphia (PA): Lippincott Williams & Wilkins, a Wolters Kluwer business;2010.
24. Lee JW, Yoon DY, Choi CS, Chang SK, Yun EJ, Seo YL, et al. Anaplastic thyroid carcinoma: computed tomographic differentiation from other thyroid masses. Acta Radiol. 2008; 49:321–327.

25. Wreesmann VB, Ghossein RA, Patel SG, Harris CP, Schnaser EA, Shaha AR, et al. Genome-wide appraisal of thyroid cancer progression. Am J Pathol. 2002; 161:1549–1556.

26. Siironen P, Hagström J, Mäenpää HO, Louhimo J, Heikkilä A, Heiskanen I, et al. Anaplastic and poorly differentiated thyroid carcinoma: therapeutic strategies and treatment outcome of 52 consecutive patients. Oncology. 2010; 79:400–408.

27. Green LD, Mack L, Pasieka JL. Anaplastic thyroid cancer and primary thyroid lymphoma: a review of these rare thyroid malignancies. J Surg Oncol. 2006; 94:725–736.

28. Takashima S, Morimoto S, Ikezoe J, Takai S, Kobayashi T, Koyama H, et al. CT evaluation of anaplastic thyroid carcinoma. AJR Am J Roentgenol. 1990; 154:1079–1085.

29. Us-Krasovec M, Golouh R, Auersperg M, Besic N, Ruparcic-Oblak L. Anaplastic thyroid carcinoma in fine needle aspirates. Acta Cytol. 1996; 40:953–958.

30. Cornillot M, Cappelaere P, Granier AM, Houcke M, Triplet I. [Cytologic diagnosis of thyroid cancer]. Lille Med. 1977; 22:366–371.
31. Luze T, Tötsch M, Bangerl I, Hittmair A, Sandbichler P, Ladurner D, et al. Fine needle aspiration cytodiagnosis of anaplastic carcinoma and malignant haemangioendothelioma of the thyroid in an endemic goitre area. Cytopathology. 1990; 1:305–310.

32. Chang TC, Liaw KY, Kuo SH, Chang CC, Chen FW. Anaplastic thyroid carcinoma: review of 24 cases, with emphasis on cytodiagnosis and leukocytosis. Taiwan Yi Xue Hui Za Zhi. 1989; 88:551–556.
33. Lee MJ, Hong SW, Chung WY, Kwak JY, Kim MJ, Kim EK. Cytological results of ultrasound-guided fine-needle aspiration cytology for thyroid nodules: emphasis on correlation with sonographic findings. Yonsei Med J. 2011; 52:838–844.

34. Moon HJ, Kwak JY, Kim EK, Kim MJ. Ultrasonographic characteristics predictive of nondiagnostic results for fine-needle aspiration biopsies of thyroid nodules. Ultrasound Med Biol. 2011; 37:549–555.

35. Sohn YM, Yoon JH, Moon HJ, Kim EK, Kwak JY. Mixed echoic thyroid nodules on ultrasound: approach to management. Yonsei Med J. 2012; 53:812–819.

36. Rinaldi P, Costantini M, Belli P, Giuliani M, Bufi E, Fubelli R, et al. Extra-mammary findings in breast MRI. Eur Radiol. 2011; 21:2268–2276.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download