Abstract
Purpose
We evaluated the prognostic value of 18F-2-fluoro-2-deoxyglucose positron emission tomography (FDG PET) in patients with resectable pancreatic cancer.
Materials and Methods
We retrospectively reviewed the medical records of pancreatic cancer patients who underwent curative resection, which included 64 consecutive patients who had preoperative FDG PET scans. For statistical analysis, the maximal standardized uptake value (SUVmax) of primary pancreatic cancer was measured. Survival time was estimated by the Kaplan-Meier method, and Cox's proportional hazard model was used to determine whether SUVmax added new predictive information concerning survival together with known prognostic factors. p<0.05 indicated statistical significance.
Results
Overall survival (OS) and disease-free survival (DFS) were respectively 42.9 months (27.6-58.2; 95% CI) and 14.9 months (10.1-19.7; 95% CI). When subjects were divided into two groups according to SUVmax with a cutoff value of 3.5, the high SUVmax group (n=32; SUVmax >3.5) showed significantly shorter OS and DFS than the low SUVmax group. Multivariate analysis of OS and DFS showed that both high SUVmax and poor tumor differentiation were independent poor prognostic factors.
Pancreatic cancer is a deadly disease and carries a poor prognosis; for all stages combined, the 5-year survival rate is less than 5%. Only 20% of patients with pancreatic cancer have resectable disease at the time of presentation, and in the event of resectable disease, the 5-year survival rate is about 20%.1,2 Prognostic factors for pancreatic cancer have been well studied, and include gender, age, size and location of the tumor, stage, lymph node metastasis, tumor grade, and serum carbohydrate antigen 19-9 (CA19-9) level.3-8
Over the past decade, 18F-2-fluoro-2-deoxyglucose positron emission tomography (FDG PET) has become established in cancer imaging. As FDG PET assesses the glucose metabolic activity of tumors, it provides useful information that cannot be obtained with other conventional imaging techniques, making it a useful imaging tool for the diagnosis and staging of pancreatic cancer, although limited sensitivity has been reported in the detection of small lesions and local lymph node metastasis.9 In addition, the metabolic activity of pancreatic tumors, measured by FDG PET usually based on a standardized uptake value (SUV), has proven useful in evaluating the prognosis of pancreatic carcinoma.10-15 Most published studies consider SUV an independent prognostic factor: higher SUV indicates a worse prognosis.
However, few studies have examined whether FDG PET is useful for the prognosis of clinical outcomes in patients with resectable pancreatic cancer. Published studies on this group of patients suffer from small numbers in subpopulation analysis or a heterogeneous group of patients with palliative resection or past history of neoadjuvant therapy.10-13
The objective of our study was to determine in a larger series of patients whether preoperative FDG PET provides prognostic information in patients with resectable pancreatic adenocarcinoma.
The institutional review board of our university approved this study and waived the informed consent requirement. Between January 2004 and August 2009, a total of 124 patients with pancreatic ductal adenocarcinoma underwent curative surgical resection at Severance Hospital. Patients were excluded from the study if they had a previous history of another malignancy, had received chemotherapy or radiotherapy before surgical resection, or had undergone palliative resection. Resectability of pancreatic cancer was determined on basis of National Comprehensive Cancer Network guidelines presented at a multidisciplinary cancer conference. Finally, 64 consecutive patients who had undergone FDG PET as a staging workup before resection were selected. We retrospectively reviewed medical records concerning age, gender, CA19-9 levels, TNM staging, type of operation, tumor size, histologic differentiation, resection margin, and adjuvant treatment.
All patients fasted for at least 4 hours before the FDG PET scan. Blood glucose levels were measured before each PET study. Patients were scanned when their plasma glucose levels were below 130 mg/dL. Scanning was initiated 60 min after the administration of FDG. Images from the neck to the proximal thigh were obtained either on an Advance PET scanner (GE Healthcare, Milwaukee, WI, USA) with a spatial resolution of 5 mm in the center of the field of view or on an Allegro PET scanner (Philips-ADAC medical systems, Cleveland, OH, USA) with a spatial resolution of 5.3 mm in the center of the field of view. When using the Advance scanner, approximately 370 MBq of FDG were injected intravenously, and an emission scan was acquired for 5 min per bed position in the two-dimensional mode. When the Allegro scanner was used, data were acquired in the three-dimensional mode after the administration of 5.18 MBq (0.14 mCi)/kg of FDG. Transmission scans (3 min per bed position) were obtained to correct for nonuniform attenuation using 68Ge and 137Cs point sources for the Advance and Allegro scanners, respectively. Transmission scans were interleaved between the multiple emission scans for the Allegro scanner. The images were reconstructed using an iterative reconstruction algorithm, that is, either the ordered-subset expectation maximization for the Advance scanner or the row action maximal-likelihood algorithm for the Allegro scanner.
All of the FDG PET images were interpreted by two experienced nuclear medicine physicians blinded to additional clinical outcomes. Focal increased standardized uptake value (SUV) was calculated as [decay-corrected activity (kBq)/mL tissue volume]/[injected FDG activity (kBq)/body mass (g)]. SUV of the pancreatic cancer was measured by manually placing a circular region of interest at the site of the maximum FDG uptake; maximum SUV (SUVmax) was used for further analysis.
Overall survival (OS) was defined as the interval from the date of curative resection to the date of death from any cause. Disease-free survival (DFS) was defined as the interval from the date of operation to the first evidence of radiological progression or to the date of death from any cause. The chi-square and Fisher exact tests were used to compare frequencies in the groups. Survival time was estimated by the Kaplan-Meier method, and differences in survival between the groups were compared using a log-rank test. Cox's proportional hazard model was used to determine whether SUVmax added new predictive information on survival. p<0.05 indicated statistical significance. Statistical analysis of the data was performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA).
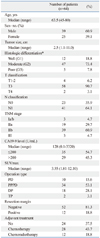
The clinical characteristics of the patient population are summarized in Table 1. Among the 64 patients studied, 34 (53.1%) underwent pylorus-preserving pancreatoduodenectomy, 18 (28.1%) distal pancreatectomy, 10 (15.6%) pancreatoduodenectomy, and two (3.1) total pancreatectomy. Forty patients had adjuvant treatment, 28 had chemotherapy, and 12 had chemoradiotherapy.
The median SUVmax of pancreatic cancers was 3.55 (range, 1.81-12.10). To compare patient characteristics descriptively according to the SUVmax, patients were divided into two groups using a cutoff of 3.5: a high SUVmax group (n=32; SUVmax >3.5) and a low SUVmax group (SUVmax ≤3.5). There were no significant differences in baseline characteristics and histologic findings between these two groups (Table 2).
The mean OS and DFS of the 64 patients were 42.9 months (27.6-58.2; 95% CI) and 14.9 months (10.1-19.7; 95% CI), respectively. Survival analysis showed that the high SUVmax group had a significantly shorter OS than the corresponding low SUVmax group (p=0.011): 23.5 months vs. 45.4 months (Fig. 1). DFS was also significantly shorter in the high SUVmax group (p=0.002): 9.2 months vs. 26.1 months.
Univariate analysis of OS showed that patients with a higher SUVmax, CA19-9 over 200 ng/mL, or poor differentiation had significantly shorter survival (p=0.011, p=0.038, and p=0.015, respectively) (Table 3). Multivariate analysis of OS showed that higher SUVmax, like poor differentiation, was a significant poor prognostic factor (p=0.025 and p=0.043, respectively) (Table 4). Univariate analysis of DFS showed that a higher SUVmax was significantly correlated with shorter DFS (p=0.002), and CA19-9 over 200 ng/mL and poor differentiation were also correlated with significantly shorter DFS (p=0.029 and p=0.000, respectively). Multivariate analysis of DFS showed that higher SUVmax and poor differentiation were independent poor prognostic factors and that poorer differentiation was also an independent prognostic factor (p=0.016 and p=0.000, respectively). However, a higher CA19-9 level did not show any significant statistical power for either OS or DFS in multivariate analysis (p=0.325 and p=0.248, respectively).
Recently, FDG PET has been widely used in cancer patients for diagnosis, staging, therapeutic monitoring, and restaging, and previous studies have reported the clinical usefulness of FDG PET in pancreatic cancer patients. In a prospective study, Kauhanen, et al.9 reported that FDG PET had a high diagnostic value in patients with pancreatic cancer compared with computed tomography (CT) and magnetic resonance imaging, with a sensitivity of 85%, specificity of 94%, positive predictive value of 94%, and negative predictive value of 85%.
Beyond the conventional role of FDG PET as a diagnostic modality, it can predict treatment responses to chemotherapy and/or radiotherapy3,16,17 and prognoses10-15 in patients with pancreatic cancer. An accelerated rate of both glucose transport and glycolysis are characteristic biochemical features of malignant transformation. FDG is a glucose analogue that is actively transported via glucose transporters (GLUT) into cells and phosphorylated by hexokinase during the first step of the glycolytic pathway. However, unlike normal glucose, phosphorylated FDG cannot continue in glycolysis and becomes trapped within the cell. Over-expression of GLUT-118,19 and hexokinase-II20 has been reported in pancreatic adenocarcinomas and differences in the biologic aggressiveness of the tumor, represented as SUV, could explain the differences in disease-free survival and overall survival in patients with pancreatic cancer after curative resection.
Some researchers have shown the clinical value of SUV during pretreatment FDG PET in predicting prognosis in patients with pancreatic cancer. For all stages combined, survival time was significantly longer in patients with lower SUV than with higher SUV.10,14 Nakata, et al.11 reported that SUV could predict prognosis in patients with unresectable disease. In a study using dual time FDG PET, a retention index of more than 10% was suggested to be a significant poor prognostic factor.13 For resectable pancreatic cancer, little information is available. SUV was significantly different between patients with resectable and unresectable diseases,21 and Sperti, et al.12 noted that a SUVmax of more than 4.0 was significantly related to poor prognosis after resection in subpopulation analysis. Recently, Lee, et al.15 suggested a glucose-corrected SUVmax of pancreatic cancer as a prognostic marker for tumor recurrence after resection. Our study also reinforced the prognostic value of FDG PET: both OS and DFS were significantly correlated with SUVmax on baseline FDG PET scans with a SUVmax cutoff of index 3.5. In a comparison of various prognostic factors by multivariate analysis, FDG PET proved to be an independent prognostic factor, where OS and DFS were significantly different above and below the cutoff value. Yet no consensus has been established on a cutoff value for SUV; different median values have been chosen as cutoff values, as in this study. Because these cutoff values vary greatly, ranging from 3.011 to 7.0,10 an absolute cutoff is not recommended for further investigations; instead, it should be determined on the basis of the individual or larger group data.
For pancreatic cancer, many prognostic factors have been suggested: tumor size, location, TNM stage, tumor differentiation, CA19-9 level, resection margin, neurovascular invasion, and adjuvant chemotherapy.3-8 Many tissue biomarkers have also been reported to be of significant prognostic value in pancreatic ductal adenocarcinoma.22 Recent comparative studies reported that conventional imaging modalities show limited accuracy in preoperative evaluation and have a tendency to understage primary pancreatic cancer.23,24 Because exact tumor stage and tissue biomarkers can only be assessed by examining the surgical specimen, such prognostic factors cannot be used to predict survival outcomes before surgery. In contrast, FDG PET is a noninvasive, convenient, and feasible modality, which is now widely used in the management of a variety of cancers. The SUV on FDG PET has several advantages over the other suggested prognostic factors mentioned above; besides its nature of preoperative assessment and whole body imaging, FDG PET does not require additional procedures for prognosis prediction. Furthermore, SUV measurement is less time-consuming and easy to calculate in everyday practice.
Our results showed that tumor differentiation was also an independent prognostic factor. CA19-9 level was significantly correlated with survival on univariate analysis, but was not an independent factor on multivariate analysis. Unlike tumor differentiation and preoperative CA19-9, the other known factors did not show statistical significance in this study. In fact, there is still controversy as to which factors can be used as independent predictors of prognosis and which have an influence on survival as well.
Curative resection is the best option for pancreatic cancer, but life expectancy is still compromised by frequent tumor recurrence. Based on our study, FDG PET would play an important role in risk stratification and thus treatment planning. We suppose that patients with high SUV, who may have a more aggressive tumor, should undergo intensive treatment as well as close follow-up. Neoadjuvant chemoradiation could also be an attractive option.
In recent years, integrated PET/CT scanners have rapidly replaced PET-only scanners. In PET/CT, probably the most relevant additional effect is that CT data frequently add specificity to the FDG PET data by providing anatomic information and thus excluding physiologic or non-specific uptakes.25,26 CT information also improves detectability of small metastatic lung nodules or lymph nodes. Published data demonstrated the superior performance of PET/CT over PET in detection, differential diagnosis, and staging of cancers,27-30 including pancreatic cancer.9,31,32 On the other hand, discrepancy with measured SUV between the two modalities may exist due to different attenuation correction methods, and a new cutoff value specific for PET/CT needs to be established for this reason.
There are several limitations in this study. Although we included a larger population with longer observation time than previous studies, the retrospective nature of the study with a still small number of cases and limited standard references limit the interpretation of the results. And as mentioned above, PET-only scanners used in this study were somewhat out-of-date. Despite these limitations, our results suggested that SUVmax is a potent prognostic factor associated with DFS, as well as OS, in patients with resectable pancreatic cancer. Now, more therapeutic options are available for pancreatic cancer with clinical evidence, and the prognostic role of FDG PET should evolve and be established with further controlled studies including a larger population.
In conclusion, SUV on FDG PET provided prognostic information in patients with resectable pancreatic cancer and may therefore play an important role in risk stratification and treatment planning prior to undertaking surgical resection.
Figures and Tables
Fig. 1
Overall survival (OS) and disease-free survival (DFS) in low SUVmax and high SUVmax groups. Tick marks on curves indicate drop-outs. (A) Cumulative survival rate was higher in the low SUVmax group (≤3.5; continuous line) than the high SUVmax group (>3.5; dotted line) (p=0.011). (B) Cumulative disease-free rate was also higher in the low SUVmax group (p=0.002). SUVmax, maximum standardized uptake value.
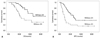
ACKNOWLEDGEMENTS
This work was supported by a research fund provided by the Yonsei Cancer Research Institute.
References
2. Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995; 221:721–731.

3. Bang S, Chung HW, Park SW, Chung JB, Yun M, Lee JD, et al. The clinical usefulness of 18-fluorodeoxyglucose positron emission tomography in the differential diagnosis, staging, and response evaluation after concurrent chemoradiotherapy for pancreatic cancer. J Clin Gastroenterol. 2006; 40:923–929.

4. Humphris JL, Chang DK, Johns AL, Scarlett CJ, Pajic M, Jones MD, et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012; 23:1713–1722.

5. Lee KJ, Yi SW, Chung MJ, Park SW, Song SY, Chung JB, et al. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J. 2013; 54:643–649.

6. Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003; 237:74–85.

7. Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000; 4:567–579.

8. Ueda M, Endo I, Nakashima M, Minami Y, Takeda K, Matsuo K, et al. Prognostic factors after resection of pancreatic cancer. World J Surg. 2009; 33:104–110.

9. Kauhanen SP, Komar G, Seppänen MP, Dean KI, Minn HR, Kajander SA, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg. 2009; 250:957–963.

10. Zimny M, Fass J, Bares R, Cremerius U, Sabri O, Buechin P, et al. Fluorodeoxyglucose positron emission tomography and the prognosis of pancreatic carcinoma. Scand J Gastroenterol. 2000; 35:883–888.

11. Nakata B, Nishimura S, Ishikawa T, Ohira M, Nishino H, Kawabe J, et al. Prognostic predictive value of 18F-fluorodeoxyglucose positron emission tomography for patients with pancreatic cancer. Int J Oncol. 2001; 19:53–58.

12. Sperti C, Pasquali C, Chierichetti F, Ferronato A, Decet G, Pedrazzoli S. 18-Fluorodeoxyglucose positron emission tomography in predicting survival of patients with pancreatic carcinoma. J Gastrointest Surg. 2003; 7:953–959.
13. Lyshchik A, Higashi T, Nakamoto Y, Fujimoto K, Doi R, Imamura M, et al. Dual-phase 18F-fluoro-2-deoxy-D-glucose positron emission tomography as a prognostic parameter in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging. 2005; 32:389–397.

14. Maemura K, Takao S, Shinchi H, Noma H, Mataki Y, Kurahara H, et al. Role of positron emission tomography in decisions on treatment strategies for pancreatic cancer. J Hepatobiliary Pancreat Surg. 2006; 13:435–441.

15. Lee SM, Kim TS, Lee JW, Kim SK, Park SJ, Han SS. Improved prognostic value of standardized uptake value corrected for blood glucose level in pancreatic cancer using F-18 FDG PET. Clin Nucl Med. 2011; 36:331–336.

16. Choi M, Heilbrun LK, Venkatramanamoorthy R, Lawhorn-Crews JM, Zalupski MM, Shields AF. Using 18F-fluorodeoxyglucose positron emission tomography to monitor clinical outcomes in patients treated with neoadjuvant chemo-radiotherapy for locally advanced pancreatic cancer. Am J Clin Oncol. 2010; 33:257–261.

17. Kuwatani M, Kawakami H, Eto K, Haba S, Shiga T, Tamaki N, et al. Modalities for evaluating chemotherapeutic efficacy and survival time in patients with advanced pancreatic cancer: comparison between FDG-PET, CT, and serum tumor markers. Intern Med. 2009; 48:867–875.

18. Higashi T, Saga T, Nakamoto Y, Ishimori T, Fujimoto K, Doi R, et al. Diagnosis of pancreatic cancer using fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET) --usefulness and limitations in "clinical reality". Ann Nucl Med. 2003; 17:261–279.

19. Reske SN, Bares R, Büll U, Guhlmann A, Moser E, Wannenmacher MF. [Clinical value of positron emission tomography (PET)in oncologic questions: results of an interdisciplinary consensus conference. Schirmerreschaft der Deutschen Gesellschaft for Nuklearmedizin]. Nuklearmedizin. 1996; 35:42–52.

20. Lyshchik A, Higashi T, Hara T, Nakamoto Y, Fujimoto K, Doi R, et al. Expression of glucose transporter-1, hexokinase-II, proliferating cell nuclear antigen and survival of patients with pancreatic cancer. Cancer Invest. 2007; 25:154–162.

21. Wakabayashi H, Nishiyama Y, Otani T, Sano T, Yachida S, Okano K, et al. Role of 18F-fluorodeoxyglucose positron emission tomography imaging in surgery for pancreatic cancer. World J Gastroenterol. 2008; 14:64–69.

22. Jamieson NB, Carter CR, McKay CJ, Oien KA. Tissue biomarkers for prognosis in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Clin Cancer Res. 2011; 17:3316–3331.

23. Bley TA, Uhl M, Simon P, Mayerle J, Ghanem NA, Geml B, et al. Diagnostic accuracy of MRI for preoperative staging of pancreatic carcinoma: tendency for understaging. In Vivo. 2005; 19:983–987.
24. Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004; 99:492–501.

25. Kapoor V, McCook BM, Torok FS. An introduction to PET-CT imaging. Radiographics. 2004; 24:523–543.

26. Hany TF, Steinert HC, Goerres GW, Buck A, von Schulthess GK. PET diagnostic accuracy: improvement with in-line PET-CT system: initial results. Radiology. 2002; 225:575–581.

27. Mottaghy FM, Sunderkötter C, Schubert R, Wohlfart P, Blumstein NM, Neumaier B, et al. Direct comparison of [18F]FDG PET/CT with PET alone and with side-by-side PET and CT in patients with malignant melanoma. Eur J Nucl Med Mol Imaging. 2007; 34:1355–1364.

28. Halpern BS, Yeom K, Fueger BJ, Lufkin RB, Czernin J, Allen-Auerbach M. Evaluation of suspected local recurrence in head and neck cancer: a comparison between PET and PET/CT for biopsy proven lesions. Eur J Radiol. 2007; 62:199–204.

29. Freudenberg LS, Rosenbaum SJ, Beyer T, Bockisch A, Antoch G. PET versus PET/CT dual-modality imaging in evaluation of lung cancer. Radiol Clin North Am. 2007; 45:639–644.

30. Kim JH, Czernin J, Allen-Auerbach MS, Halpern BS, Fueger BJ, Hecht JR, et al. Comparison between 18F-FDG PET, in-line PET/CT, and software fusion for restaging of recurrent colorectal cancer. J Nucl Med. 2005; 46:587–595.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download