Abstract
Purpose
The presence of gastrointestinal (GI) cancer and its treatment might aggravate patient nutritional status. Malnutrition is one of the major factors affecting the postoperative course. We evaluated changes in perioperative nutritional status and risk factors of postoperative severe malnutrition in the GI cancer patients.
Materials and Methods
Nutritional status was prospectively evaluated using patient-generated subjective global assessment (PG-SGA) perioperatively between May and September 2011.
Results
A total of 435 patients were enrolled. Among them, 279 patients had been diagnosed with gastric cancer and 156 with colorectal cancer. Minimal invasive surgery was performed in 225 patients. PG-SGA score increased from 4.5 preoperatively to 10.6 postoperatively (p<0.001). Ten patients (2.3%) were severely malnourished preoperatively, increasing to 115 patients (26.3%) postoperatively. In gastric cancer patients, postoperative severe malnourishment increased significantly (p<0.006). In univariate analysis, old age (>60, p<0.001), male sex (p=0.020), preoperative weight loss (p=0.008), gastric cancer (p<0.001), and open surgery (p<0.001) were indicated as risk factors of postoperative severe malnutrition. In multivariate analysis, old age, preoperative weight loss, gastric cancer, and open surgery remained significant as risk factors of severe malnutrition.
Conclusion
The prevalence of severe malnutrition among GI cancer patients in this study increased from 2.3% preoperatively to 26.3% after an operation. Old age, preoperative weight loss, gastric cancer, and open surgery were shown to be risk factors of postoperative severe malnutrition. In patients at high risk of postoperative severe malnutrition, adequate nutritional support should be considered.
Nutritional status is an important factor affecting patient compliance to a therapeutic approach. Malnutrition has been shown to be closely associated with postoperative outcomes of morbidity and mortality. It may compromise a patient's immunity and increase their susceptibility to infection in postoperative periods.1-3
Among hospitalized patients, about 10 to 57% are malnutritioned, and 12 to 42% of these patients have severe malnutrition requiring preoperative nutritional support.4-6 Severe malnutrition is usually associated with increases in rate of infection, length of hospital stay and complications.6-8 Cancer patients are at a high risk of severe malnutrition, and about 30 to 40% of them are accompanied by severe weight loss and malnorishment.9,10
According to guidelines from the European Society of Parenteral and Enteral Nutrition (ESPEN) and American Society of Parenteral and Enteral Nutrition, perioperative nutritional support should be considered in cancer patients. Further, for severely malnourished patients, they recommend performing surgery after administering preoperative nutritional support for more than 7 days.11,12
The patient generated subjective global assessment (PG-SGA) comprises six components addressing changes in weight and food intake, gastrointestinal symptoms, physical activity and functional capacity, metabolic demand related to medical diagnosis and physical examination. Nutritional status is categorized into three groups: stage A (well nourished), stage B (moderately malnourished) and stage C (severely malnourished). Recently, the PG-SGA has been accepted as a simple and quick nutritional assessment tool in hospitalized cancer patients.13
Despite the above, few have studied the prevalence of perioperative malnutrition and changes in nutritional status among gastrointestinal cancer patients. Accordingly, we attempted to evaluate changes in nutritional status according PG-SGA score as well as risk factors for postoperative severe malnutrition in the perioperative period in patients with gastrointestinal cancer requiring surgical treatment.
This was a prospective observation study designed to assess short-term changes in nutritional status in the perioperative period among gastrointestinal cancer patients.
Five hundred patients who were to undergo an operation for gastrointestinal cancer were prospectively evaluated in regards to their nutritional status using the PG-SGA from April to September 2011. This study protocol was approved by the institutional review board and conducted after obtaining informed consent from the patients.
The inclusion criteria were as follows: 1) gastric cancer or colorectal cancer patients, 2) 20-80 years of age, and 3) patients requiring elective surgery for cancer treatment. Patients were dropped from the study after the first nutritional assessment in instances of 1) a canceled operation, 2) open and closure due to advanced cancer, 3) prolonged hospital stay (>30 days), and 4) early (<10 days) or late (>35 days) visit to the outpatient clinic after being discharged. A total of 435 patients were evaluated for nutritional status postoperatively (Fig. 1).
Demographics such as sex, age, height, weight, body mass index, diagnosis, name of operation, type & route of operation, perioperative nutritional support, preoperative treatment, and staging were checked by reviewing the patients' medical records.
Two dietitians assessed patient nutritional status on the day before the operation and at the 1st visit to the out-patient clinic (OPD) after being discharge using scored PG-SGA. We categorized patients into three groups according to PG-SGA stage and calculated PG-SGA score. Severe malnutrition was defined as PG-SGA stage C.
Data analyses were performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were expressed as mean and standard deviation. Factors affecting PG-SGA categories were analyzed with chi-test, and ANOVA was used to compare PG-SGA score among the three groups according to the SGA categories. Multivariate analysis was performed with a logistic regression model to discover risk factors of postoperative severe malnutrition.
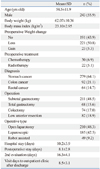
In total, 435 patients were analyzed through this study. There were 243 men (55.9%) and the mean age of the patients was 58 years old. Sixty-four percent (n=279) had gastric cancer, 21.1% (n=92) had colon cancer, and 14.7% (n=64) had rectal cancer. Subtotal gastrectomy was performed in 43.5% (n= 211), total gastrectomy in 15.6% (n=68), colectomy in 17.0% (n=74) and low anterior resection in 18.9% (n=82). The operation was performed thru open laparotomy in 211 (48.6%) patients, as well as laparoscopic- and robot-assisted surgery in 185 (42.5%) and 49 (9.2%) patients, respectively. Open laparotomy was performed in 190 (68.1%) of the gastric cancer patients and 20 (13.8%) of the colorectal cancer patients. The length of total hospital stay was 10 days and the length of postoperative hospital stay was 8 days. A secondary nutritional assessment was performed on the 16th post-operation day, which was the 8th day after discharge (Table 1). The lengths of postoperative hospital stay were 7.3 days in the PG-SGA group A, 8.0 days in group B, and 9.0 days in group C (p=0.001).
Body weight significantly decreased from 62.1 kg to 58.9 kg after operation (p<0.001). Weight loss in the preoperative and postoperative periods was 1.96 kg and 3.29 kg, respectively. PG-SGA score increased from 4.5 to 10.6 after operation (p<0.001). According to PG-SGA rating, the proportion of severely malnourished patients increased from 2.3% to 26.2% after operation (p<0.001) (Table 2, Fig. 2).
The proportion of severely malnourished stomach cancer patients increased significantly from 2.2% to 29.7% (p=0.006). However, among colorectal cancer patients, such changes were not significant (p=0.157) (Table 3).
Univariate analysis showed (Table 3) that male gender, old age (>60 years), stomach cancer, open laparotomy, high preoperative PG-SGA score (>4) and preoperative weight loss were related to postoperative malnutrition. Cancer stage did not affect the incidence of postoperative severe malnutrition (Table 4).
Most of the colon cancer patients underwent minimally invasive surgery, although the type of operation did not affect postoperative nutritional status (p=0.291) in these patients. However, among stomach cancer patients, there was a significant difference in postoperative nutritional status according to the type of operation (open vs. minimally invasive group, 32.1% vs. 24.7%, p=0.001).
Multivariate analysis was performed to identify possible risk factors for postoperative severe malnutrition. Variables entered into the analysis included age, gender, diagnosis, methods of the operation (open or minimally invasive), and preoperative weight loss. Multivariate analysis revealed that old age (>60 years old), preoperative weight loss, open surgery and stomach cancer were significant risk factors for postoperative severe malnutrition (Table 5).
Gastrointestinal (GI) cancer patients are considered at high risk for malnutrition, and the prevalence of preoperative malnutrition among them has been reported as nearly 10-20%.2,3,14-16 Furthermore, after surgical treatment, appetite and diet are known to decline during recovery, leading to malnutrition. Nutritional status can take up to a year to recover after an operation.15 Preoperative severe malnutrition is known to affect postoperative outcomes, such as length of hospital stay. Up to now, however, there have been no reports on immediate nutritional changes after operation in gastrointestinal cancer patients.
This study showed that the incidence of severe malnutrition preoperatively was very low (2.3%), but increased to 26.2% postoperation. In the present study, all gastrointestinal cancer patients scheduled for an operation, except for emergency situations, were enrolled during the study period. Most of the patients (323, 74.3%) had early stage disease, so nutritional status therein might not have changed from that before being diagnosed with cancer. However, 49.7% (n=216) had a PG-SGA score higher than four requiring nutritional intervention. Furthermore, 12.4% (n=54) had a score higher than nine requiring nutritional therapy. Most of those with a higher score were older age and experienced weight loss associated with cancer. Unfortunately, we were unable to evaluate postoperative outcomes such as length of hospital stay and newly developed infectious complications with regards to preoperative malnutrition because of its low incidence in the study cohort. Although the length of hospital stay was longer in patients with postoperative malnutrition, we did not evaluate the factors affecting the length of stay. Postoperative hospital stay is affected by various factors, including a patient's condition, comorbidities, postoperative complications, and economic standing.
Postoperative malnutrition was more frequent in gastric cancer patients (29.7%) than in colorectal cancer patients (19.9%). Resection of the stomach reduces reservoir function, and may cause early fullness and a smaller diet. Therefore, postgastrectomy patients can experience significant weight loss and postoperative malnutrition. Interestingly, in the present study, there was no difference in the incidence of postoperative malnutrition between patients who received total gastrectomy and patients who received subtotal gastrectomy, showing different results from a previous study.17 The difference in our patients compared with those in the other study was the longer period of study, which was more than 3 months.17,18 No reports have published concerning short-term changes in nutritional status in the perioperative period nor immediate changes in nutritional status in regards to long-term outcomes. Yet assessment of long-term outcomes of such patients according to postoperative nutritional status is warranted. In colorectal cancer patients, diets may not change, and their nutritional status could be more stable than stomach cancer patients. However, as observed in this study, severe malnutrition increased from 2.6% to 19.9% in colorectal cancer patients, indicating the need for awareness of their postoperative nutritional status.
Minimally invasive surgery is known to be helpful in postoperative recovery due to minimal stress and less nutritional impairment.19 In this study, minimally invasive surgery was superior in postoperative nutritional aspects. Accordingly, we suggest the application of minimally invasive surgery in patients at high risk for postoperative malnutrition. Moreover, good nutritional status may be helpful to postoperative recovery. In stomach cancer patients, postoperative malnutrition was higher in open surgery groups, but this was not different in colorectal cancer patients. Open surgery is a more invasive and destructive procedure causing stress and a greater inflammatory response, and may require longer recovery times after the operation.
Preoperative weight loss is a risk factor for postoperative malnutrition. Accordingly, it is a parameter in several nutritional screening and assessment tools. Gastrointestinal cancer induces changes in taste and oral habitus, leading to reduced oral intake. Thus, weight loss is usually associated with cancer. Changes in appetite and food intake, as well as development of gastrointestinal symptoms also contribute to weight loss in cancer patients. These are included in the PG-SGA, so we did not analyze these separately. In this study, higher preoperative PG-SGA score (>4) was related to postoperative malnutrition. Therefore, preoperative changes in food intake and gastrointestinal symptoms can be considered as risk factors for postoperative severe malnutrition.
In the present study, cancer TNM stage did not affect changes in nutritional status. This indicates that the major causes of postoperative nutritional change are associated with recovery from surgery not cancer itself. However, advanced cancer patients may involve poor nutritional status for longer periods.10 This study did not analyze postoperative outcomes associated with preoperative and postoperative nutritional status.
For high nutritional risk patients, preoperative nutritional intervention is very important to reducing postoperative morbidity and severe malnutrition.1-3,11,12,17,20 In this study, we did not provide preoperative nutritional support, but almost all patients (>99%) had been infused peripheral parenteral nutrition (PPN) products during the postoperative period. Postoperative PPN did not prevent postoperative weight loss, but we could not evaluate the efficiency of the peripheral nutritional support. According to recent clinical guidelines for surgical patients, parenteral nutrition is beneficial in undernourished patients in whom enteral nutrition is not feasible or not tolerated or in patients with postoperative complications impairing gastrointestinal function who are unable to receive and absorb adequate amounts of oral/enteral feeding for at least 7 days (ESPEN guidelines on parenteral nutrition: surgery). Therefore, greater concern for the adequacy and efficiency of postoperative nutritional support with regard to oral/enteral and parenteral nutrition is warranted.
There are few reports on immediate perioperative changes in nutritional status. This study only compared early changes in nutritional status and risk factors for early postoperative severe malnutrition. We could not show the postoperative outcomes associated with nutritional status due to the low incidence of preoperative malnutrition. However, this study provides basic data for comparing long-term results according to early postoperative nutritional status. Additionally, it may help highlight the importance of nutritional assessment and intervention in GI cancer patients at high risk for postoperative severe malnutrition.
In conclusion, the incidence of postoperative severe malnutrition increased to 26.2% in gastrointestinal cancer patients. Older patients, patients with preoperative weight loss, stomach cancer patients, and patients who underwent open laparotomy were shown to be at high risk for postoperative severe malnutrition. Further studies are needed to evaluate the efficacy of aggressive nutritional support and intervention in patients at high risk of postoperative severe malnutrition and the impact of postoperative severe malnutrition on long-term oncologic outcomes in the gastrointestinal cancer patients.
Figures and Tables
 | Fig. 2Changes in perioperative nutritional status. Preoperative PG-SGA scores increased postoperatively (A), the incidence of the severe malnutrition (PG-SGA category C) also increased postoperatively (B). PG-SGA, patient generated subjective global assessment. |
Notes
References
1. Bozzetti F, Gianotti L, Braga M, Di Carlo V, Mariani L. Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr. 2007; 26:698–709.

2. Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet. 2010; 23:393–401.

3. Rey-Ferro M, Castaño R, Orozco O, Serna A, Moreno A. Nutritional and immunologic evaluation of patients with gastric cancer before and after surgery. Nutrition. 1997; 13:878–881.

4. Dzieniszewski J, Jarosz M, Szczygieł B, Długosz J, Marlicz K, Linke K, et al. Nutritional status of patients hospitalised in Poland. Eur J Clin Nutr. 2005; 59:552–560.

5. Pham NV, Cox-Reijven PL, Wodzig WK, Greve JW, Soeters PB. SGA and measures for muscle mass and strength in surgical Vietnamese patients. Nutrition. 2007; 23:283–291.

6. Waitzberg DL, Caiaffa WT, Correia MI. Hospital malnutrition: the Brazilian national survey (IBRANUTRI): a study of 4000 patients. Nutrition. 2001; 17:573–580.

7. Correia MI, Campos AC. ELAN Cooperative Study. Prevalence of hospital malnutrition in Latin America: the multicenter ELAN study. Nutrition. 2003; 19:823–825.

8. Naber TH, Schermer T, de Bree A, Nusteling K, Eggink L, Kruimel JW, et al. Prevalence of malnutrition in nonsurgical hospitalized patients and its association with disease complications. Am J Clin Nutr. 1997; 66:1232–1239.

9. Bozzetti F. SCRINIO Working Group. Screening the nutritional status in oncology: a preliminary report on 1,000 outpatients. Support Care Cancer. 2009; 17:279–284.

10. Ollenschläger G, Viell B, Thomas W, Konkol K, Bürger B. Tumor anorexia: causes, assessment, treatment. Recent Results Cancer Res. 1991; 121:249–259.

11. Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F, et al. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr. 2009; 28:378–386.

12. Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr. 2006; 25:224–244.

13. Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002; 56:779–785.

14. Oh CA, Kim DH, Oh SJ, Choi MG, Noh JH, Sohn TS, et al. Changes of the preoperative and postoperative nutritional statuses in patients with gastric cancer and assessment of the nutritional factors that are correlated with short-term postoperative complications. J Korean Gastric Cancer Assoc. 2010; 10:5–12.

15. Ryu SW, Kim IH. Comparison of different nutritional assessments in detecting malnutrition among gastric cancer patients. World J Gastroenterol. 2010; 16:3310–3317.

16. Guo W, Ou G, Li X, Huang J, Liu J, Wei H. Screening of the nutritional risk of patients with gastric carcinoma before operation by NRS 2002 and its relationship with postoperative results. J Gastroenterol Hepatol. 2010; 25:800–803.

17. Ryan AM, Healy LA, Power DG, Rowley SP, Reynolds JV. Short-term nutritional implications of total gastrectomy for malignancy, and the impact of parenteral nutritional support. Clin Nutr. 2007; 26:718–727.

18. Kiyama T, Mizutani T, Okuda T, Fujita I, Tokunaga A, Tajiri T, et al. Postoperative changes in body composition after gastrectomy. J Gastrointest Surg. 2005; 9:313–319.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download