Abstract
Purpose
The dipeptidyl peptidase IV (DPPIV) gene family exhibits multiple functions and is involved in the pathogenesis of various diseases. It has attracted pharmaceutical interest in the areas of metabolic disorders as well as cancer. However, clinicopathologic significance of DPPIV family in colorectal cancer is not fully understood.
Materials and Methods
The clinical relevance of DPPIV and DPP10 expression was determined by immunohistochemical staining, and by assessing its clinicopathologic correlation in 383 colorectal cancer patients with known clinical outcomes.
Results
DPPIV was not expressed in normal colon mucosa, but it showed luminal expression in 52 of the 383 colorectal cancers (13.5%). DPPIV expression in tumors was associated with right-sided location of the colon (p=0.010) and more advanced tumor stage (p=0.045). DPP10 was expressed in normal colonic mucosa, but its expression varied in primary colorectal cancer tissues. Loss of DPP10 expression was found in 11 colorectal cancers (CRCs) (2.9%), and multivariate analysis showed that loss of DPP10 expression was an independent factor for poor patient prognosis (p=0.008).
Colorectal cancer is the third most common malignancy in Western countries1 and in South Korea.2 Despite remarkable advances in diagnostic and therapeutic modalities, 20% of patients are still diagnosed with colorectal cancer at a far advanced stage, with distant metastasis, and these patients have a 5-year survival rate of only 12%.3 In order to reduce cancer incidence and mortality rate, early detection with appropriate therapeutic management is necessary. Molecular biomarker for cancer may be helpful for diagnosis, prediction of prognosis or therapeutic target.4
The dipeptidyl peptidase IV (DPPIV) gene family has garnered increasing interest due to its diverse functions related to pathogenesis of inflammatory, metabolic, and endocrine disorders, and cancer progression.5 DPPIV inhibitors have merged as successful therapeutic targets for type 2 diabetes mellitus and the application of dipeptidyl peptidase inhibitors in pathogenic conditions, such as cancer, has been carefully considered.5 The DPPIV gene family belongs to the prolyl oligopeptidase family of enzymes5 and comprises proteins with DPP enzymatic activity and DPP4 structural homologues.6 To date, eight DDP peptidases have been identified, DPP3, DPPIV, DPP6, DPP7, DPP8, DPP9, DPP10, and fibroblast activation protein.7 DPPIV is the best-studied member of this family, and had been shown to be involved in cell differentiation, adhesion, immunomodulation, and apoptosis, all critical for controlling neoplastic transformation.8-10 Also, decreased expression of DPPIV has been reported in various malignancies.8,10-13
DPP10 is a newly cloned peptidase with structural similarity to DPPIV.14 DPP10 lacks DPP4-like enzyme activity due to a point mutation in its active site, where a serine is replaced by a glycine residue.14 It binds to specific voltage-gated potassium channels, altering their structures and biophysical properties,15 but the cellular functions of DPP10 remain unknown. DPP10 has been linked to asthma susceptibility by several genome-wide association studies,16,17 and recently impaired expression of DPP10 gene in malignant mesothelioma18 and nasopharyngeal carcinoma has been reported.19
In the present study, we evaluated protein expression of DPPIV and DPP10 in colorectal cancer tissue using immunohistochemistry and compared their expression with clinical relevance. We found that loss of DPP10 expression in primary colorectal cancer is significantly associated with poor survival outcomes. We further evaluated the level of protein expression of DPP10 using western blot in selected colorectal cancer tissues.
For immunohistochemical analysis, tissue microarray blocks were constructed from formalin-fixed paraffin-embedded tissues of 383 patients with colorectal cancer who underwent surgical resection at the National Cancer Center of Korea in 2003. The clinical charts and pathological reports of those 383 patients were reviewed. These 383 patients included 152 women and 231 men, with a mean age of 58 years (range, 25 to 86 years), and a mean follow-up time of 64 months (range: 2-74 months). Liver metastasis was identified in 90 patients, 69 with synchronous and 21 with metachronous metastasis, with a mean metastasis duration of 19 months (range, 1 to 67 months). All patients were classified according to World Health Organization criteria and staged according to the 2009 American Joint Committee on Cancer.
For Western blot analysis, tissue samples were obtained from 10 colorectal cancer patients with synchronous liver metastases, who underwent surgical resection at the National Cancer Center, Korea. We obtained four tissue samples from each patient: normal colonic mucosa, primary colorectal cancer, normal liver, and liver metastasis.
Informed consent was obtained from all of the enrolled patients. This study was approved by the Institutional Review Board of the National Cancer Center of Korea.
Immunostaining was performed using the labeled streptavidin-biotin complex method. After antigen retrieval, the samples were incubated with mouse monoclonal anti-DPPIV (CD26) antibody (ready-to-use; MBL, Nagoya, Japan), rabbit polyclonal anti-DPP10 antibody (dilution 1 : 500; Abcam, Cambridge, UK), and mouse monoclonal anti-carcinoembryonic antigen (CEA) antibody (clone C-II, dilution 1 : 200; DAKO, Carpinteria, CA, USA). As a negative control, the tissue sections were incubated with Tris-buffered saline alone, without the primary antibody. DPPIV expression was mainly identified in luminal borders or as an intraluminal secretory pattern. The staining results for DPPIV were classified as positive if any secretory expression was discernible. There was no reported immunostaining criterion of DPP10 loss. At first, DPP10 staining was quantified using H score20 by multiplying the staining intensity by the percentage of stained tumor cells. Staining intensity was classified as 1 for weak, 2 for moderate, or 3 for strong staining. H scores ranged from 0 to 300. Then, in order to determine a cutoff value of H score, Kaplan-Meier analysis was performed according to groups with DPP10 H scores of 0-20, 21-50, 51-100, 101-150, 151-200, 201-250, and >250. As for CEA, the staining pattern was classified into 0, no expression; 1+, focal expression along the luminal border; 2+, partial membranous expression along the luminal and cytoplasmic borders; or 3+, diffuse membranous and cytoplasmic expression. For statistical analysis, 3+ was defined as 'high' and 0 to 2+ were defined as 'low'. All immunohistochemical staining results were evaluated independently by two pathologists (HSP and HJC).
Western blot analysis was performed as described.21 Briefly, 4000×g supernatant fractions of cell homogenates containing equivalent amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA), followed by blocking for 2 h at 4℃ in 1% Tween 20-TBS buffer containing 1.5% non-fat dry milk (Bio-Rad, Richmond, CA, USA) and 1 mM MgCl2. The membranes were incubated for 2 h at room temperature with primary antibody against DPP10 (Abcam, UK), washed three times for 15 minutes each with blocking solution and incubated with diluted horseradish peroxidase-conjugated secondary antibody (SouthernBiotech, Birmingham, AL, UK) for 1 hour at room temperature. The membranes were washed three times for 15 min each with blocking solution and incubated with WEST-ZOL (plus) chemiluminescence reagent (iNtRON Biotechnology, Seoul, Korea) for 1 minute and exposed to film (Kodak Blue XB-1, Rochester, NY, USA).
The χ2-test was used to evaluate the relationship between immunohistochemical expression of DPPIV and DPP10 and clinicopathological features. Cancer-specific or disease free survival was estimated using the Kaplan-Meier method and compared with the log-rank test. The prognostic value of protein expression was determined by multivariate analysis using the Cox proportional hazards regression model. The non-parametric Mann-Whitney U test was used to calculate between-group differences in DPPIV and DPP10 expression on Western blotting. p-values <0.05 were considered statistically significant. All statistical analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA).
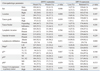
DPPIV was not expressed in normal colon mucosa (Fig. 1). Fifty-two (13.5%) out of 383 primary colorectal cancers showed DPPIV expression (Fig. 1). DPPIV expression in tumors was significantly associated with right-sided location of the colon (p=0.008) and more advanced T stage (p=0.047) (Table 1), but there was no significant relationship with other clinical parameters including distant metastasis or survival benefit.
DPP10 was expressed in normal large intestinal mucosa, but its expression varied in colorectal cancers (Fig. 2). The mean±standard deviation of H score for colorectal cancers was 157.19±77.69 (range 0 to 295). In order to determine the cutoff score of DPP10 for clinical significance, Kaplan-Meier analysis was performed according to groups with DPP10 H scores of 0-20, 21-50, 51-100, 101-150, 151-200, 201-250, and >250. There was a gap in clinical outcomes between tumors with a DPP10 score of 20 or less and those with a DPP10 score above 20. Therefore, loss of DPP10 was defined as a DPP10 H score of 20 or less. According to this criterion, 11 (2.9%) out of 383 primary colorectal cancers showed loss of expression. Loss of DPP10 was significantly associated with female preponderance (p=0.023) and diffuse CEA expression in the primary tumor (p=0.037) (Table 1). However, loss of DPP10 expression was not associated with pT, pN, or pM stage. Univariate survival analyses showed that patients with tumors retaining DPP10 expression had significantly longer cancer-specific (p=0.013) and progression-free (p=0.0475) survival than patients with tumors that did not express DPP10 (Fig. 3). Multivariate analyses showed that loss of DPP10 expression was an independent poor prognostic factor for cancer-related death (p=0.001) and disease recurrence (p=0.002) (Table 2).
In order to confirm down-regulation of DPP10 in colorectal cancer compared to normal tissue, we performed western blot analysis of DPP10 in quadruplet samples of colorectal cancer with synchronous liver metastasis. DPP10 protein has two isoforms: isoform 1 with a molecular size of 90934 Da, and isoform 2 with a molecular size of 90186 Da.7 Western blotting of fresh samples of matched normal colon-primary colon cancer-normal liver-liver metastasis taken from 10 patients with colorectal cancer revealed various levels of expression of DPP10 protein in these samples (Fig. 4A). However, the level of expression of DPP10 was significantly lower in primary tumor than in normal colonic mucosa (p=0.018) (Fig. 4B).
In the present study, we identified DPP10 as a candidate protein related to colorectal cancer progression, and to our knowledge, this is the first study on the role of DPP10 in colorectal cancer. Western blot analysis showed that DPP10 expression was decreased in tumors compared with normal tissue. Few studies to date have assessed the association between DPP10 and cancer. A large deletion in the DPP10 gene, producing a truncated fusion transcript, was reported in patients with malignant mesothelioma, and overall survival was significantly longer in patients with tumors that did than did not express DPP10 transcripts.18 DPP10 gene has reported to be down-regulated in nasopharyngeal cancer.19 Similarly, we found that loss of DPP10 immunostaining in primary colorectal tumors was associated with significantly poorer cancer-specific and disease-free survival outcomes, although the number of cases with DPP10 loss was small. The exact role of DPP10 in colorectal cancer is unknown, but it may affect tumor cell survival or growth as it modulates potassium channel function, which is crucial in regulating cell proliferation, cell cycle progression, and apoptosis.14,22 Loss of DPP expression by a primary carcinoma was not associated with liver metastasis in the present study.
DPPIV was originally identified as a T cell differentiation antigen (CD26) and it is 110-kDa glycoprotein with an extracellular domain possessing ectopeptidase activity.21 This enzyme cleaves X-Pro dipeptides from the N-terminal end of peptides and proteins, inactivating or degrading mitogenic growth factors and neuropeptides.9 DPPIV expression had been identified in various cell types including on lymphocytes, melanocytes, epithelial brush borders of the intestine, kidney, and pancreas duct, and liver hepatocytes.8,23,24 DPPIV is multifunctional and has merged as a new therapeutic target for type 2 diabetes.5 DPPIV expression has been reported to be decreased in various malignancies, including nonsmall cell lung cancer cell lines,11 melanoma cells,8 metastatic prostate cancer cells,10 and high grade endometrial carcinomas.12 Restoration of DPPIV expression in cancer cell lines resulted in a change of cell phenotype, to a nonmalignant or differentiated phenotype,8,10,11 suggesting that DPPIV may play a role in tumor suppression. However, it has been also reported to be upregulated in lung adenocarcinoma, 25 prostate carcinoma,26 and thyroid carcinoma.27 In colorectal cancer, variable expression of adenosine deaminase complexing protein (the identical protein of DPPIV) has been reported28 and recently, decreased serum DPPIV level has been reported in colorectal cancer patients, compared to benign or non-neoplastic colorectal disease groups.29 In the present study, we did not observe DPPIV expression in normal colonic epithelial cells, but focal aberrant expression of DPPIV was present in colorectal cancer tissues as the secretory form within cancer glandular lumen, but the expression was not relevant to clinicopathologic findings except for right-side location (p=0.010). Fric, et al.30 reported similar results showing a higher expression of DPPIV in sporadic right colon cancer than in left colon cancer. However, our results do not suggest that DPPIV plays a role in colorectal cancer, in terms of the frequency and localization of the protein expression. Indeed, intracellular expression of DPPIV was rare in our cases. Taking into consideration that DPPIV can be originated from various cell types, DPPIV level in serum may not reflect the DPPIV level secreted from colorectal cancer cells.
To our knowledge, this is the first study describing the role of DPP10 in colorectal cancer with its prognostic significance. However, our study has limitations, including the retrospective evaluation, small number of positive cases, and the lack of more distinct functional or molecular evidence for the role of DPP10 in colorectal cancer progression. In conclusion, our results suggest that DPP10 might play a role in the progression of colorectal cancer, and may be an independent prognostic marker in patients with colorectal cancer. Further studies to clarify the underlying molecular mechanism of the role of DPP10 in colorectal cancer are needed.
Figures and Tables
Fig. 1
Immunohistochemistry of DPPIV in primary colorectal cancers (×200). (A) Normal crypt epithelium did not express DPPIV protein. (B) Some colorectal cancers showed intraluminal expression of DPPIV. (C) Rare colorectal cancers showed focal cytoplasmic expression of DPPIV. DPPIV, dipeptidyl peptidase IV.

Fig. 2
Immunohistochemistry of DPP10 in primary colorectal cancers (×200). (A) Normal crypt epithelium showed mild DPP10 expression in cytoplasm. (B) Most colorectal cancers retained DPP10 expression. (C) Some colorectal cancers showed loss of DPP10 expression. DPP, dipeptidyl peptidase.

Fig. 3
Kaplan-Meier survival curves according to the DPP10 expression in 383 colorectal cancer patients. Loss of DPP10 expression in primary tumor was significantly associated with worse cancer-specific (A) and progression-free (B) survival. DPP, dipeptidyl peptidase.

Fig. 4
Western blot of DPP10 in 10 colorectal cancer patients with synchronous liver metastasis. (A) Western blotting of matched normal colon, primary colorectal cancer, and liver metastasis from 10 selected patients showed variable expression of DPP10. (B) Level of expression of DPP10 in primary colorectal cancer was significantly decreased. N, normal; T, tumor; DPP, dipeptidyl peptidase; OD, optical density.

ACKNOWLEDGEMENTS
HSP assessed immunohistochemical results and prepared and edited the manuscript. HYY and KHK performed the proteomic and Western blot analyses. JWP, BCK, JYB, SYK, and DYK participated in the design of the study and helped to draft the manuscript. HJC designed and coordinated the study, performed immunohistochemical and statistical analyses, and edited the manuscript. All authors read and approved the final manuscript.
This study was supported by a grant from the National Cancer Center of Korea (NCC-0910160), and by the Converging Research Center Program funded by the Ministry of Education, Science and Technology (Project No. 1131150). This research was supported by the Converging Research Center Program funded by the Ministry of Science, ICT and Future Planning (Project No. 2013K000271).
References
1. Rastogi T, Hildesheim A, Sinha R. Opportunities for cancer epidemiology in developing countries. Nat Rev Cancer. 2004; 4:909–917.

2. Korea Central Cancer Resitry MoHaW, Republic of Korea. 2008 Annual Report of the Korea Central Cancer Registry. 2010.
3. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011; 61:212–236.
4. Bacolod MD, Barany F. Molecular profiling of colon tumors: the search for clinically relevant biomarkers of progression, prognosis, therapeutics, and predisposition. Ann Surg Oncol. 2011; 18:3694–3700.

5. Yu DM, Yao TW, Chowdhury S, Nadvi NA, Osborne B, Church WB, et al. The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J. 2010; 277:1126–1144.

6. Zagha E, Ozaita A, Chang SY, Nadal MS, Lin U, Saganich MJ, et al. DPP10 modulates Kv4-mediated A-type potassium channels. J Biol Chem. 2005; 280:18853–18861.

7. HUGO/GNC. Available at http://www.geneontology.org.
8. Wesley UV, Albino AP, Tiwari S, Houghton AN. A role for dipeptidyl peptidase IV in suppressing the malignant phenotype of melanocytic cells. J Exp Med. 1999; 190:311–322.

9. Boonacker E, Van Noorden CJ. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol. 2003; 82:53–73.

10. Wesley UV, Tiwari S, Houghton AN. Role for dipeptidyl peptidase IV in tumor suppression of human non small cell lung carcinoma cells. Int J Cancer. 2004; 109:855–866.

11. Sedo A, Krepela E, Kasafírek E, Kraml J, Kadlecová L. Dipeptidyl peptidase IV in the human lung and spinocellular lung cancer. Physiol Res. 1991; 40:359–362.
12. Khin EE, Kikkawa F, Ino K, Kajiyama H, Suzuki T, Shibata K, et al. Dipeptidyl peptidase IV expression in endometrial endometrioid adenocarcinoma and its inverse correlation with tumor grade. Am J Obstet Gynecol. 2003; 188:670–676.

13. Tan EY, Mujoomdar M, Blay J. Adenosine down-regulates the surface expression of dipeptidyl peptidase IV on HT-29 human colorectal carcinoma cells: implications for cancer cell behavior. Am J Pathol. 2004; 165:319–330.

14. Qi SY, Riviere PJ, Trojnar J, Junien JL, Akinsanya KO. Cloning and characterization of dipeptidyl peptidase 10, a new member of an emerging subgroup of serine proteases. Biochem J. 2003; 373(Pt 1):179–189.

15. Li HL, Qu YJ, Lu YC, Bondarenko VE, Wang S, Skerrett IM, et al. DPP10 is an inactivation modulatory protein of Kv4.3 and Kv1.4. Am J Physiol Cell Physiol. 2006; 291:C966–C976.

16. Michel S, Liang L, Depner M, Klopp N, Ruether A, Kumar A, et al. Unifying candidate gene and GWAS Approaches in Asthma. PLoS One. 2010; 5:e13894.

17. Wu H, Romieu I, Shi M, Hancock DB, Li H, Sienra-Monge JJ, et al. Evaluation of candidate genes in a genome-wide association study of childhood asthma in Mexicans. J Allergy Clin Immunol. 2010; 125:321–327.

18. Bueno R, De Rienzo A, Dong L, Gordon GJ, Hercus CF, Richards WG, et al. Second generation sequencing of the mesothelioma tumor genome. PLoS One. 2010; 5:e10612.

19. Xiong S, Wang Q, Zheng L, Gao F, Li J. Identification of candidate molecular markers of nasopharyngeal carcinoma by tissue microarray and in situ hybridization. Med Oncol. 2011; 28:Suppl 1. S341–S348.

20. Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol. 2008; 26:1059–1065.

21. Boonacker EP, Wierenga EA, Smits HH, Van Noorden CJ. CD26/DPPIV signal transduction function, but not proteolytic activity, is directly related to its expression level on human Th1 and Th2 cell lines as detected with living cell cytochemistry. J Histochem Cytochem. 2002; 50:1169–1177.

22. Allen M, Heinzmann A, Noguchi E, Abecasis G, Broxholme J, Ponting CP, et al. Positional cloning of a novel gene influencing asthma from chromosome 2q14. Nat Genet. 2003; 35:258–263.

23. McCaughan GW, Wickson JE, Creswick PF, Gorrell MD. Identification of the bile canalicular cell surface molecule GP110 as the ectopeptidase dipeptidyl peptidase IV: an analysis by tissue distribution, purification and N-terminal amino acid sequence. Hepatology. 1990; 11:534–544.

24. Boonacker E, Elferink S, Bardai A, Fleischer B, Van Noorden CJ. Fluorogenic substrate [Ala-Pro]2-cresyl violet but not Ala-Pro-rhodamine 110 is cleaved specifically by DPPIV activity: a study in living Jurkat cells and CD26/DPPIV-transfected Jurkat cells. J Histochem Cytochem. 2003; 51:959–968.

25. Asada Y, Aratake Y, Kotani T, Marutsuka K, Araki Y, Ohtaki S, et al. Expression of dipeptidyl aminopeptidase IV activity in human lung carcinoma. Histopathology. 1993; 23:265–270.

26. Wilson MJ, Ruhland AR, Quast BJ, Reddy PK, Ewing SL, Sinha AA. Dipeptidylpeptidase IV activities are elevated in prostate cancers and adjacent benign hyperplastic glands. J Androl. 2000; 21:220–226.
27. Aratake Y, Kotani T, Tamura K, Araki Y, Kuribayashi T, Konoe K, et al. Dipeptidyl aminopeptidase IV staining of cytologic preparations to distinguish benign from malignant thyroid diseases. Am J Clin Pathol. 1991; 96:306–310.

28. ten Kate J, van den Ingh HF, Khan PM, Bosman FT. Adenosine deaminase complexing protein (ADCP) immunoreactivity in colorectal adenocarcinoma. Int J Cancer. 1986; 37:479–485.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download