Abstract
Purpose
Four polymorphisms, -765G>C, -1195G>A, 8473T>C, and Val511Ala, in the cyclooxygenase-2 (COX-2) gene were identified to be associated with colorectal cancer (CRC) risk. However, the results are inconsistent. The objective of this meta-analysis was to evaluate the association between these four polymorphisms and the risk of CRC.
Materials and Methods
All eligible case-control studies published up to December 2012 on the association between the four polymorphisms of COX-2 and CRC risk were identified by searching PubMed and Web of Science. The CRC risk associated with the four polymorphisms of the COX-2 gene was estimated for each study by odds ratio (OR) together with its 95 % confidence interval (CI), respectively.
Results
A total of 15 case-control studies were included. Overall, no evidence has indicated that the -1195A allele, -765C allele, 8473C allele, and 511Ala allele are associated with susceptibility to CRC (-1195G>A: OR=1.11, 95 % CI: 0.82-1.51, p=0.78; -765G>C: OR=1.08, 95 % CI: 0.96-1.21, p=0.07; 8473T>C: OR=1.03, 95 % CI: 0.89-1.18, p=0.91; Val511Ala: OR=0.71, 95 % CI: 0.46-1.09, p=0.94). However, stratified analysis with ethnicity indicated that individuals with -765GC or GC/CC genotypes had an increased risk of CRC among Asian populations (GC vs. GG: OR=1.05, 95 % CI: 0.87-1.28, p=0.03; GC+CC vs. GG: OR=1.08, 95 % CI: 0.96-1.21, p=0.07).
Colorectal cancer (CRC) is a common digestive malignancy, the incidence of which is just lower than gastric and esophageal cancer. With continuous improvement in living standards, general health has improved greatly; however, the incidence of CRC has markedly ascended. Molecular epidemiology has confirmed that tumorigenesis is close related to interactions between one's genetic background and the environment. Most CRC occurrences arise due to interactions between environmental and genetic factors.1
Cyclooxygenase-2 (COX-2) is an inducible isoform of COX enzymes that converts arachidonic acid to prostaglandins, which are potent mediators of inflammation. COX-2 is related to several biological processes, including carcinogenesis, cell proliferation, angiogenesis, and mediating immune suppression. A growing body of evidence has shown that increased expression of COX-2 is closely related to malignant progression.2-5 Moreover, it is reported that selective COX-2 inhibitors could prevent carcinogenesis.6 The human COX-2 gene, mapped to chromosome 1q25.2-q25.3, is 8.3 kb in length and contains 10 exons and 9 introns. There are different polymorphism sites in the COX-2 gene,7,8 and four of these polymorphisms, rs20417 (-765G>C), rs689466 (-1195G>A), rs5275 (8473T>C) and rs5273 (Val511Ala), are the most extensively studied polymorphisms in CRC.
Recently, 15 studies have investigated the association between these four polymorphisms and the susceptibility of CRC in diverse populations.9-23 However, the results remain controversial. To better address the association between COX-2 polymorphisms and CRC risk, we performed a meta-analysis of all eligible studies to evaluate the association between these four polymorphisms of the COX-2 gene and CRC risk.
A literature research was conducted using PubMed and Web of Science up to December 2012 without language restrictions. Relevant studies were identified using the terms: ('cyclooxygenase-2 or COX-2 or PTGs2') and ('genetic polymorphism or polymorphisms or single-nucleotide polymorphism') and ('colorectal cancer/neoplasms or colon cancer/neoplasms or rectal cancer/neoplasms'). The search was restricted to humans. Additional studies were identified by a hand search of references of original or review articles on this topic. If data or data subsets were published in more than one article, only the publication with the largest sample size was included.
Studies were included if they met the following criteria: 1) studies that evaluated the association between the four polymorphisms (-765G>C, -1195G>A, 8473T>C, and Val511Ala) and CRC, 2) a case-control study design, and 3) had detailed genotype frequency of cases and controls or could be calculated from the article text. The major exclusion criteria were: 1) case-only study, case reports, and review articles, 2) studies without the raw data of the four genotypes of COX-2, 3) studies that compared the COX-2 variants in familial adenomatous polyposis, or colorectal adenoma, and 4) studies that investigated COX-2 variants as marks for response to therapy.
The two investigators (Wang J and Guo XF) independently extracted data according to the inclusion criteria. Disagreement was resolved by discussion between them. If they could not reach a consensus, an expert (Dong WG) was consulted to resolve the dispute and a final majority decision was made. For each study, the following data was collected: the first author's name, year of publication, country of origin, ethnicity, number of genotyped cases and controls, and minor allele frequency in the controls. Patient ethnicity was categorized as Asian, Caucasian, and African-American.
Meta-analysis was performed using the Cochrane Collaboration RevMan 5.0 (Copenhagen, 2008) and STATA package version 9.2 (Stata Corporation, College Station, TX, USA) software. We calculated odd ratios corresponding to a 95 % confidence interval (95 % CI) to assess the strength of association between the four polymorphisms of the COX-2 gene and CRC risk. Heterogeneity assumption was checked by a χ2-based Q test.24 We also quantified the effect of heterogeneity by I2 test. When a significant Q test (p<0.1) or I2 >50 % indicated heterogeneity across studies, the random effects model was used,25 or else the fixed effects model was used.26 Before the effect estimation of COX-2 polymorphisms in colorectal cancer, we tested whether genotype frequencies of controls were in Hardy-Weinberg equilibrium (HWE) using χ2 test. Four comparison genetic models were used to assess the association: the dominant model (the combined variant homozygote and heterozygote versus the wild-type homozygote), the recessive model (the variant homozygote versus the combined heterozygote and wild-type homozygote), the heterozygote comparison (heterozygote versus the wild-type homozygote), and the homozygote comparison (variant homozygote versus the wild-type homozygote). Stratification analyses were performed on ethnicity. Analysis of sensitivity was performed to evaluate the stability of the results. Finally, potential publication bias was investigated using Begg's funnel plot and Egger's regression test.27,28 p< 0.05 was regarded as statistically significant.
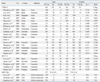
The search strategy retrieved 99 potentially relevant studies. According to the inclusion criteria, 15 studies with full-text were included in this meta-analysis and 84 studies were excluded. The flow chart of study selection in summarized in Fig. 1. As shown in Table 1, there were 11 case-control studies with 3432 cancer cases and 5286 controls concerning -765G>C polymorphism, 5 case-control studies with 1854 cancer cases and 2950 controls concerning -1195G>A, 5 case-control studies with 1827 cancer cases and 2853 controls concerning 8473T>C, and 3 case-control studies with 493 cancer cases and 784 controls concerning Val511Ala. Three ethnicities were addressed: five studies focused on Asian populations, 9,11-13,19 seven on Caucasian populations,10,14-18,20 and three on African-American populations.21-23 The distribution of genotypes in the controls was consistent with the HWE for all selected studies, except for one study for -765G>C,11 and three studies for Val511Ala,21-23 the PHWE of which were not available.
Eleven studies reported the association between COX-2 -765G>C polymorphism and susceptibility to CRC. Overall, there was no significant difference in COX-2 -765G>C genotype distribution between CRC and controls [GC+CC vs. GG (OR=1.08, 95 % CI: 0.96-1.21, p=0.07); CC vs. GG (OR=1.11, 95 % CI: 0.77-1.60, p=0.96); GC vs. GG (OR=1.05, 95 % CI: 0.87-1.28, p=0.03); CC vs. GC+GG (OR=1.09, 95 % CI: 0.76-1.56, p=0.85)] (Table 2, Fig. 2). In the subgroup analysis by ethnicity, results were similar in the Caucasian population, while significantly increased risk was found in those of Asian descent [GC+CC vs. GG (OR=1.41, 95 % CI: 1.15-1.75, p=0.39); GC vs. GG (OR=1.48, 95 % CI: 1.15-1.90, p=0.24)] (Table 2, Fig.3).
The -1195G>A COX-2 polymorphism analysis, fitting into five studies, revealed that there was no significant difference in COX-2 -1195G>A genotype distribution between CRC and controls in the Caucasian population [GA+AA vs. GG (OR=1.11, 95 % CI: 0.82-1.51, p=0.78); AA vs. GG (OR=1.14, 95 % CI: 0.84-1.56, p=0.69); GA vs. GG (OR=1.05, 95 % CI: 0.76-1.44, p=0.91); AA vs. GA+GG (OR=1.08, 95 % CI: 0.96-1.22, p=0.27)] (Table 2, Fig. 4A).
Five studies reported an association between COX-2 8473T>C polymorphism and susceptibility to CRC, all patients came from Caucasian populations. No association was found between 8473C allele and susceptibility to CRC [TC+CC vs.TT (OR=1.03, 95 % CI: 0.89-1.18, p=0.91); CC vs.TT (OR=0.96, 95 % CI: 0.76-1.21, p=0.81); TC vs.TT (OR=1.04, 95 % CI: 0.90-1.21, p=0.85); CC vs. TC+TT (OR=0.93, 95 % CI: 0.75-1.16, p=0.74)] (Table 2, Fig. 4B).
There were three studies that reported an association between COX-2 Val511Ala polymorphism and susceptibility to CRC, and all patients came from African-American populations. The results showed that no association between 511Ala allele and susceptibility to CRC [Val/Ala+Ala/Ala vs. Val/Val (OR=0.71, 95 % CI: 0.46-1.09, p=0.94)] (Table 2, Fig. 4C).
Sensitivity analysis was performed by sequential omission of individual studies. For -765G>C polymorphism, The estimated pooled odd ratio did not change after excluding the study that was not in HWE. For the other polymorphisms, the significance of pooled OR in all individual analyses was not influenced excessively by omitting any single study. The above analysis indicated that the results were stable and statistically robust.
We used Begg's funnel plot and Egger's test to address potential publication bias in the available literature. The publication bias of the meta-analysis on the association between COX-2 polymorphisms and susceptibility to CRC was detected for all four polymorphisms in a dominant model. The shape of the funnel plots did not reveal any evidence of funnel plot asymmetry. Egger's test also showed that there was no statistical significance for the evaluation of publication bias (P765G>C=0.904, P1195G>A=0.136, P8473T>C=0.361, PVal511Ala=0.485) (Fig. 5).
Evidence suggests that COX-2 plays an important role in carcinogenesis.29,30 The specific function of COX-2 in the formation of prostaglandins makes it a strong candidate for increasing susceptibility to common cancers such as colorectal cancer, gastric cancer and other cancers.31 Eberhart, et al.32 reported that more than 85 % of human colon cancers have elevated levels of COX-2. Regular use of COX-2 inhibitor has been shown to decrease the relation risk of developing colorectal cancer.33 It is reported that polymorphisms may alter the expression of COX-2 and thereby modulate the risk for various cancers. Although the exact molecular mechanism is still unclear, several polymorphisms in COX-2 have been reported previously, and the results are still controversial.
The present meta-analysis included 3432 cancer cases and 5286 controls concerning -765G>C polymorphism, 1854 cancer cases and 2950 controls concerning -1195G>A, 1827 cancer cases and 2853 controls concerning 8473T>C, and 493 cancer cases and 784 controls concerning Val511Ala in the coding regions of COX-2. And we explored the role of these four potentially functional polymorphisms of COX-2 in susceptibility to CRC. The COX-2 -765G>C polymorphism is within the promoter region, which appears to disrupt a stimulatory protein1 binding site, and leads to a 30 % reduction of COX-2 promoter activity in vitro.34 In this study, no significant association between COX-2 -765G>C and the risk of CRC under all four genetic models in overall comparisons were observed. However, in the subgroup analysis by ethnicity, COX-2 -765C allele was significantly associated with an increasing risk of CRC in Asian populations, but not for Caucasian populations. The results may due to ethnic differences in genetic backgrounds and the environment in which they lived. The COX-2 -1195G>A polymorphism, also located in the promoter region, which contains several key cis-acting regulatory elements and may play important roles in the regulation of COX-2 transcription.35 This meta-analysis included five studies, all of which came from the Caucasian population, and found that COX-2 -1195G>A polymorphism was not significantly related to a risk of CRC. The COX-2 8473T>C polymorphism is located in the 3'-untranslated region, which contains highly-conserved adenine-uracil-rich elements. This motif is involved in the regulation of COX-2 production by acting both as an mRNA instability determinant and a translation inhibitory element.36-38 However, we also found no association between COX-2 8473T>C and risk of CRC. The Val511Ala polymorphism, identified only in African-Americans, showed nonsignificant relevance to risk of CRC in this study. In short, the results may be explained by different ethnic groups. Interactions with other genetic variants are possible reasons. In addition, gene-environmental factors may also explain the discrepancies. However, because only few studies on European populations were included, this result should be interpreted with caution, and more studies are needed.
Some limitations of this meta-analysis should be addressed. First, because of incomplete raw data or publication limitations, some relevant studies could not be included in our analysis. Second, the number of published studies was not sufficiently large, and some studies of small size may not have enough statistical power to explore the real association. Third, some misclassifications may be occurred, which would influence our results. Fourth, the overall outcomes were based on unadjusted estimates, and some potentially suspected factors such as age, sex, smoking and environmental factors were not analysis, so the result should be cautiously interpreted.
In summary, this meta-analysis sought to provide evidence for associations between -765G>C, -1195G>A, 8473T>C, and Val511Ala polymorphisms and CRC risk, and discerned that -765G>C may lead to an increased risk in those of Asian descent. However, no evidence indicated that -1195G>A and 8473T>C were associated with susceptibility to CRC in Caucasians, nor was Val511Ala in African-Americans. However, large and well-designed studies are warranted to validate our findings.
Figures and Tables
 | Fig. 2Meta-analysis of the association between -765G>C polymorphism and susceptibility to colorectal cancer. (A) Dominant model. (B) GC vs. GG. CI, confidence interval. |
 | Fig. 3Subgroup analysis of -765G>C polymorphism by ethnicity. (A) dominant model. (B) GC vs. GG. CI, confidence interval. |
 | Fig. 4Meta-analysis of the association between COX-2 polymorphism and susceptibility to colorectal cancer in the dominant model. (A) -1195G>A. (B) 8473T>C. (C) Val511Ala. CI, confidence interval. |
 | Fig. 5Begg's funnel plot for publication bias. Each point represents a separate study for the indicated association. Log [OR], natural logarithm of OR. Horizontal line, mean effect size. (A) -765G>C. (B) -1195G>A. (C) 8473T>C. (D) Val511Ala. OR, odds ratio. |
Table 2
Summary of ORs for COX-2 Polymorphism and Colorectal Cancer Risk

CI, confidence interval; COX-2, cyclooxygenase-2; OR, odds ratio; SNP, single-nucleotide polymorphism; Ht+VR vs. WT Ho, dominant model; VR Ho vs. Ht+WT Ho, recessive model.
*There were five studies in the dominant model, four in the other models.
†Random-effects model was used when the p for heterogeneity test was ≤0.05, otherwise the fixed-effect model was used.
‡Test for heterogeneity.
References
1. Hutter CM, Chang-Claude J, Slattery ML, Pflugeisen BM, Lin Y, Duggan D, et al. Characterization of gene-environment interactions for colorectal cancer susceptibility loci. Cancer Res. 2012; 72:2036–2044.

2. Trifan OC, Hla T. Cyclooxygenase-2 modulates cellular growth and promotes tumorigenesis. J Cell Mol Med. 2003; 7:207–222.

3. Gately S. The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metastasis Rev. 2000; 19:19–27.
4. Oshima H, Oshima M. The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models. J Gastroenterol. 2012; 47:97–106.

5. van Rees BP, Ristimäki A. Cyclooxygenase-2 in carcinogenesis of the gastrointestinal tract. Scand J Gastroenterol. 2001; 36:897–903.

6. Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? Lancet Oncol. 2003; 4:605–615.

7. Fritsche E, Baek SJ, King LM, Zeldin DC, Eling TE, Bell DA. Functional characterization of cyclooxygenase-2 polymorphisms. J Pharmacol Exp Ther. 2001; 299:468–476.
8. Wiesner GL, Platzer P, Buxbaum S, Lewis S, MacMillen M, Olechnowicz J, et al. Testing for colon neoplasia susceptibility variants at the human COX2 locus. J Natl Cancer Inst. 2001; 93:635–639.

9. Hamajima N, Takezaki T, Matsuo K, Saito T, Inoue M, Hirai T, et al. Genotype Frequencies of Cyclooxygenease 2 (COX2) Rare Polymorphisms for Japanese with and without Colorectal Cancer. Asian Pac J Cancer Prev. 2001; 2:57–62.
10. Cox DG, Pontes C, Guino E, Navarro M, Osorio A, Canzian F, et al. Polymorphisms in prostaglandin synthase 2/cyclooxygenase 2 (PTGS2/COX2) and risk of colorectal cancer. Br J Cancer. 2004; 91:339–343.

11. Koh WP, Yuan JM, van den, Lee HP, Yu MC. Interaction between cyclooxygenase-2 gene polymorphism and dietary n-6 polyunsaturated fatty acids on colon cancer risk: the Singapore Chinese Health Study. Br J Cancer. 2004; 90:1760–1764.

12. Tan W, Wu J, Zhang X, Guo Y, Liu J, Sun T, et al. Associations of functional polymorphisms in cyclooxygenase-2 and platelet 12-lipoxygenase with risk of occurrence and advanced disease status of colorectal cancer. Carcinogenesis. 2007; 28:1197–1201.

13. Xing LL, Wang ZN, Jiang L, Zhang Y, Xu YY, Li J, et al. Cyclooxygenase 2 polymorphism and colorectal cancer: -765G>C variant modifies risk associated with smoking and body mass index. World J Gastroenterol. 2008; 14:1785–1789.

14. Iglesias D, Nejda N, Azcoita MM, Schwartz S Jr, González-Aguilera JJ, Fernández-Peralta AM. Effect of COX2 -765G>C and c.3618A>G polymorphisms on the risk and survival of sporadic colorectal cancer. Cancer Causes Control. 2009; 20:1421–1429.

15. Thompson CL, Plummer SJ, Merkulova A, Cheng I, Tucker TC, Casey G, et al. No association between cyclooxygenase-2 and uridine diphosphate glucuronosyltransferase 1A6 genetic polymorphisms and colon cancer risk. World J Gastroenterol. 2009; 15:2240–2244.

16. Hoff JH, te Morsche RH, Roelofs HM, van der Logt EM, Nagengast FM, Peters WH. COX-2 polymorphisms -765G-->C and -1195A-->G and colorectal cancer risk. World J Gastroenterol. 2009; 15:4561–4565.
17. Andersen V, Ostergaard M, Christensen J, Overvad K, Tjønneland A, Vogel U. Polymorphisms in the xenobiotic transporter Multidrug Resistance 1 (MDR1) and interaction with meat intake in relation to risk of colorectal cancer in a Danish prospective case-cohort study. BMC Cancer. 2009; 9:407.

18. Pereira C, Pimentel-Nunes P, Brandão C, Moreira-Dias L, Medeiros R, Dinis-Ribeiro M. COX-2 polymorphisms and colorectal cancer risk: a strategy for chemoprevention. Eur J Gastroenterol Hepatol. 2010; 22:607–613.

19. Daraei A, Salehi R, Mohamadhashem F. PTGS2 (COX2) -765G>C gene polymorphism and risk of sporadic colorectal cancer in Iranian population. Mol Biol Rep. 2012; 39:5219–5224.

20. Siezen CL, Bueno-de-Mesquita HB, Peeters PH, Kram NR, van Doeselaar M, van Kranen HJ. Polymorphisms in the genes involved in the arachidonic acid-pathway, fish consumption and the risk of colorectal cancer. Int J Cancer. 2006; 119:297–303.

21. Lin HJ, Lakkides KM, Keku TO, Reddy ST, Louie AD, Kau IH, et al. Prostaglandin H synthase 2 variant (Val511Ala) in African Americans may reduce the risk for colorectal neoplasia. Cancer Epidemiol Biomarkers Prev. 2002; 11:1305–1315.
22. Goodman JE, Bowman ED, Chanock SJ, Alberg AJ, Harris CC. Arachidonate lipoxygenase (ALOX) and cyclooxygenase (COX) polymorphisms and colon cancer risk. Carcinogenesis. 2004; 25:2467–2472.

23. Sansbury LB, Millikan RC, Schroeder JC, North KE, Moorman PG, Keku TO, et al. COX-2 polymorphism, use of nonsteroidal anti-inflammatory drugs, and risk of colon cancer in African Americans (United States). Cancer Causes Control. 2006; 17:257–266.

24. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997; 127:820–826.

26. Mantel N, Haenzel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959; 22:719–748.
27. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–1101.

28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.

29. Wang D, Mann JR, DuBois RN. The role of prostaglandins and other eicosanoids in the gastrointestinal tract. Gastroenterology. 2005; 128:1445–1461.

30. Leahy KM, Koki AT, Masferrer JL. Role of cyclooxygenases in angiogenesis. Curr Med Chem. 2000; 7:1163–1170.

31. Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta. 2000; 1470:M69–M78.

32. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994; 107:1183–1188.

33. Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001; 1:11–21.

34. Papafili A, Hill MR, Brull DJ, McAnulty RJ, Marshall RP, Humphries SE, et al. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol. 2002; 22:1631–1636.
35. Dixon DA. Regulation of COX-2 expression in human cancers. Prog Exp Tumor Res. 2003; 37:52–71.
36. Shaw G, Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986; 46:659–667.

37. Gou Q, Liu CH, Ben-Av P, Hla T. Dissociation of basal turnover and cytokine-induced transcript stabilization of the human cyclooxygenase-2 mRNA by mutagenesis of the 3'-untranslated region. Biochem Biophys Res Commun. 1998; 242:508–512.

38. Dixon DA, Kaplan CD, McIntyre TM, Zimmerman GA, Prescott SM. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3'-untranslated region. J Biol Chem. 2000; 275:11750–11757.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download