Abstract
Purpose
This study tried to identify novel gastric autoimmune antigens that might be involved in aggravating the atrophic gastritis among patients with Helicobacter pylori infection using two-dimensional immunoblotting analysis.
Materials and Methods
Proteins from gastric mucosal antrectomy specimens and AGS cells (gastric adenocarcinoma cell lines derived from a Caucasian patient who had received no prior therapy) were 2-dimensionally immunoblotted separately with a pool of 300 sera from H. pylroi-infected patients at Gyeongsang National University Hospital.
Results
Thirty-eight autoantigenic proteins including alcohol dehydrogenase [NADP+], alpha enolase, gastrokine-1, gastric triacylglycerol lipase, heat shock 70 kDa protein 1, and peroxiredoxin-2 were identified in the gastric mucosal tissue. Fourteen autoantigenic proteins including programmed cell death 6-interacting protein, serum albumin and T-complex protein 1 subunit gamma were identified in the AGS cells. Albumin, alpha-enolase, annexin A3, cytoplasmic actin 1, heat shock cognate 71 kDa protein and leukocyte elastase inhibitor were commonly observed autoantigenic proteins in both gastric mucosal tissue and AGS cells. Alpha-enolase, glutathione S-transferase P, heat shock cognate 71 kDa protein, heat shock 70 kDa protein 1, human mitochondrial adenosine triphosphate synthase (ATP) subunit beta, mitochondrial 60 kDa heat shock protein, peroxiredoxin-2, 78 kDa glucose-regulated protein precursor, tyrosine-protein phosphatase non-receptor type 11 and Tryptophan-Aspartic acid (WD) repeat-containing protein 1 showed 60% or higher amino acid positivity.
Conclusion
These newly identified gastric autoimmune antigens might be useful in the control and prevention of gastroduodenal disorders, and might be valuable in breaking the vicious circle that exists in gastroduodenal disorders if their pathophysiological roles could be understood in the progress of chronic atrophic gastritis, gastroduodenal ulcers, intestinal metaplasia, and gastric carcinogenesis.
Chronic gastritis is usually divided into type A and B. Type A gastritis (fundal gastritis) is characterized by diffuse atrophic changes, intestinal metaplasia, and inflammatory changes of the gastric body and fundus, and associated with achlorhydria and pernicious anemia. The autoantiboides against parietal cells, proton pump (H+,K+-ATPase) and intrinsic factors are considered to play the main pathogenetic roles.1,2 On the other hand, type B gastritis (antral gastritis) shows mucosal atrophic changes, intestinal metaplasia, and inflammatory changes of gastric antrum. Initially, lesions in the stomach are sparse, with progression to larger lesions of the gastric body through the gastric lesser curvature. Autoantibodies had not been considered as etiologies until recently. Helicobacter pylori was identified as an etiologic agent of type B gastritis by Marshall and Warren in 1984.3 It also became known to play a main role in the pathogenesis of gastric ulcer, duodenal ulcer, gastric carcinoma and gastric mucosa associated lymphoid tissue lymphoma.4-6 In addition to chronic and active inflammatory responses in gastric lamina propria induced by H. pylori infection,7,8 anti-gastric autoimmune antibodies have also been suggested to play a role in the process from H. pylori infection in a young child to gastric carcinoma in a late life through gastric mucosal atrophy and intestinal metaplasia.9 Anti-parietal cell antibody is documented in 50% of patients with chronic type B gastritis, and the inflammatory responses by the autoimmune reaction are implicated with the development and progression of chronic type B gastritis.10
The human immune system recognizes invading bacteria and their derived materials as new antigens and initiates the removal of the antigens using specific or non-specific immune responses. H. pylori typically inhabit the gastric mucosa or junctions between epithelial cells, which are regions poorly surveyed by the host immune defense system. Persistence of infections can lead to a host hypersensitivity response that progressively destroys host tissue with time.11 H. pylori infection can produce both humoral and cell-mediated immune reactions, and chronic repetitive infection can stimulate an autoimmune gastritis because of bacterial molecular mimicry with gastric tissue molecules.12
Koreans are infected with H. pylori from early infancy and most are carriers by 10-years-of-age.13,14 Therefore, some adult Koreans with H. pylori infection might have high titers of anti-H. pylori antibodies and its cross-reactive anti-gastric tissue autoantibodies. The presence of an anti-gastric cell immune response in the absence of serum antibodies for Lewis x and Lewis y has been reported,15,16 and the observed atrophic phenomena are not confined to the parietal cells of gastric body, but are found diffusely and multifocally throughout the gastric mucosa. Therefore, there may be as-yet unidentified antoantigenic proteins in gastric mucosa that play important roles in the pathophysiology of H. pylori-induced gastric carcinogenesis. In the present study, we performed proteomics investigations of anti-gastric autoantibody profiles in the sera of 300 H. pylori infected Korean adults.
This study was approved by the Gyeongsang National University Hospital Institutional Review Board (GNUHIRB-5413).
The gastric antrectomy surgical specimen was obtained from a 29-year-old man with a gastric perforation because of gastric ulcer. The specimen was moderately infected with H. pylori and showed severe chronic gastritis with mild activity supported by many lymphoid follicles and neutrophil infiltrations on pathologic examination. There was no intestinal metaplasia. The specimen had been kept deeply frozen in the Gyeongsang National University Hospital (GNUH) Biobank of Korea. Following specimen retrieval, the muscular layer was cut off of the specimen using a knife. The AGS human adenocarcinoma epithelial cell line (CRL 1739; American Type Culture Collection, Manassas, VA, USA) was obtained from Korean Cell Line Bank. The cells were grown in RPMI 1640 medium (Lonza, Walkersyille, MD, USA) with 10% fetal bovine serum (Lonza) and 100 units/mL of gentamicin at 37℃ and 5% CO2. A pool of 300 sera obtained from patients, confirmed H. pylori-infected by immunoblotting at GNUH, was also taken from the same biobank. The serum samples were collected from healthy candidates who visited GNUH for medical check-up and 300 H. pylori-infected sera of the collected serum samples were pooled.
To homogenize the gastric mucosa, 0.15 g of the tissue was solubilized in 1 mL of 50 mM Tris-HCL (pH 7.2), containing 10 µL of protease inhibitor and 10 µL of 100 mM ethylenediaminetetraacetic acid (EDTA). Homogenized gastric mucosa and collected AGS cells were separately lysed in solubilization buffer, composed of 8 M urea, 4% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 100 mM dithiothreitol (DTT), and 40 mM Tris-HCl (pH 7.2). Proteins derived from the extracts of gastric mucosa and AGS cells were separated by two-dimensional-PAGE (2-DE). In brief, isoelectrofocusing was performed on 17-cm pH 5-8 IPG strips (Bio-Rad, Hercules, CA, USA). The strips were rehydrated for 13 hours in a 300 µL of solution consisting of 7 M urea, 2 M thiourea, 2% CHAPS, 100 mM DTT, and 0.5% ampholyte (Bio-rad) containing 60 µg of the protein sample. Strips were electrofocused at 60000 Vh, with a maximum of 10000 V at 20℃ using the Protean IEF cell (Bio-rad). Prior to the second dimension, strips were incubated in equilibration buffer (375 mM Tris-HCl, pH 8.8, 6 M urea, 2% SDS, 30% glycerol) with 2% DTT for 10 min and then with 2.5% iodoacetamide in the same buffer without DTT for 10 min. The separation was performed on 7.5-17.5% SDS-PAGE gradient gels using a PROTEAN II xi 2-D cell (Bio-rad).
The separated proteins on other gels were transferred to nitrocellulose membranes. Following blocking with 1% BSA in TBST buffer (Tris-buffered saline containing 0.1% Tween 20) for 1 hour at 37℃, the membranes were subjected to IgG immunoblotting using the 1 : 10 diluted mixed sera of H. pylori infected patients for 45 minutes at 37℃. After three times washes with TBST (Tris-Buffered Saline and Tween 20) buffer, the membranes were incubated with alkaline phosphatase-conjugated rabbit anti-human IgG as a secondary antibody (gamma chain specific; DAKO A/S, Glostrup, Denmark) at a 1 : 1000 dilution for 30 minutes at 37℃. After five times washes, the membranes were processed using an enzyme reaction with 5-bromo-4-chloro-3-indolyl phosphate, p-toluidine salt and nitro blue tetrazolium chloride in alkaline phosphate buffer. The protein patterns in some gels were visualized directly by silver17 or Coomassie blue staining.
Gel images were obtained by scanning the silver or Coomassie blue stained gels with the Fluor-S Multi Image system (Bio-rad) incorporating the PDQUEST program.
The excised silver stained protein spots were destained with the chemical reducers [30 mM K3Fe(CN)6, 100 mM Na2S2 O3·5H2O] and in-gel digested.18 Protein identification was repeated three times using spots from different gels. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) was carried out using a Voyager Biospectrometry Workstation (PE Biosystem, Foster City, CA, USA) with the following parameters: 20 kV accelerating voltage, 75% grid voltage, 0.02% guide wire voltage, 150 ns delay, and a mass gate from 800-3500. Peptide mass fingerprints were searched using the Protein Prospector Package program MS-FIT (http://prospector.ucsf.edu/ucsfhtml/msfit.htm; ProteinPtospector V 5.8.0) and NCBI database (SwissProt. 2011.01.11). In addition, the amino acid sequences of the peptides were deduced and searched for homogeneity with H. pylori peptides using the BLASTp program of NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Forty-four autogantigenic protein spots were identified from gastric mucosa by 2-DE and immunoblotting (Fig. 1, Table 1). Of these, retinal dehydrogenase 1, gastric lipase, gastrokine-1, and alpha enolase had several overlapping spots. Thirty-eight novel proteins were identified by immunoblotting: ahydrolase domain-containing protein 14B, actin-related protein 3, aflatoxin B1 aldehyde reductase member 3, alcohol dehydrogenase [NADP+], alpha enolase, alpha-1-antitrypsin, annexin A3, annexin A4, carbonic anhydrase 1, carbonic anhydrase 2, creatine kinase B-type, cytoplasmic actin 1, cytoplasmic malate dehydrogenase, fructose-1,6-bisphosphatase 1, gastrokine-1, gastric triacylglycerol lipase, glutathione S-transferase Mu 3, glutathione S-transferase P, heat shock cognate 71 kDa protein, heat shock 70 kDa protein 1, hemoglobin subunit beta, leukocyte elastase inhibitor, L-lactate dehydrogenase B chain, mitochondrial aconitate hydratase, mitochondrial ATP synthase subunit beta, mitochondrial dihydrolipoyl dehydrogenase, mitochondrial 60 kDa heat shock protein, peroxiredoxin-2, peroxiredoxin-6, protein disulfide-isomerase A3, Rab GDP dissociation inhibitor beta, retinal dehydrogenase 1, selenium-binding protein 1, serum albumin, 78 kDa glucose-regulated protein, Uridine Diphophate (UDP)-glucose 4-epimerase, vitamin D-binding protein, and Tryptophan-Aspartic acid (WD) repeat-containing protein 1.
From AGS cells, 19 autoantigenic protein spots were present in 2-D immunoblotting. Three of them (actin cytoplasmic 1, T-complex protein 1 subunit gamma, and album) had several spots. From AGS cells, 14 unique antigens were identified: alpha-enolase, annexin A3, cytoplasmic actin 1, cytoplasmic isocitrate dehydrogenase [NADP], heat shock cognate 71 kDa protein, leukocyte elastase inhibitor, mitochondrial glycine amidinotransferase, programmed cell death 6-interacting protein, serum albumin, T-complex protein 1 subunit gamma, T-complex protein 1 subunit alpha, tyrosine-protein phosphatase non-receptor type 11, T-complex protein 1 subunit theta and T-complex protein 1 subunit epsilon (Fig. 2, Table 2). Of these, tyrosine-protein phosphatase non-receptor type 11, T-complex protein 1 subunit alpha, T-complex protein 1 subunit gamma, T-complex protein 1 subunit theta, T-complex protein 1 subunit epsilon, cytoplasmic NADP, mitochondrial glycine amidinotransferase, and programmed cell death 6-interacting protein were noted only in gels of AGS cells.
Heat shock cognate 71 kDa protein, cytoplasmic actin 1, alpha-enolase, annexin A3, albumin, and leukocyte elastase inhibitor were present in both gastric antral mucosa tissue and AGS cells (Fig. 3).
Amino acid identity and positivity between autoantigens derived from gastric mucosa and AGS cells and proteins derived from H. pylori were identified. Thirty-seven proteins had some identity and positivity of amino acid sequences (Table 3). Of these proteins, mitochondrial ATP synthase subunit beta (H. pylori 26695; F0F1 ATP synthase subunit beta), WD repeat-containing protein 1 (DNA-directed RNA polymerase subunit beta), mitochondrial 60 kDa heat shock protein (chaperonin GroEL), tyrosine-protein phosphatase non-receptor type 11 (glutamine synthetase; J99), 78 kDa glucose-regulated protein (molecular chaperone DnaK), heat shock cognate 71 kDa protein (molecular chaperone DnaK), heat shock 70 kDa protein 1 (molecular chaperone DnaK), peroxiredoxin-2 (alkyl hydroperoxide reductase), alpha-enolase (phosphopyruvate hydratase), and glutathione S-transferase P (putative type II DNA modification enzyme) had over 60% amino acid positivity, compared with amino acid sequences of proteins from H. pylori. Amino acid sequence of mitochontrial ATP synthase subunit beta had the highest identity (68%) and positivity (80%), compared with that of F0F1 ATP synthase subunit beta from H. pylori 26695.
Ten common antigens derived from both gastric mucosa and AGS cells had amino acid identity and positivity with proteins of H. pylori. Gastric lipase, gastrokine-1, selenium-binding protein 1, alpha-1-antitrypsin precursor, Rab GDP dissociation inhibitor beta, L-lactate dehydrogenase B chain, abhydrolase domain-containing protein 14B, and hemoglobin subunit beta had no amino acid identity or positivity from proteins of H. pylori.
This study examined the involvement of autoimmune antigenic proteins in the course of gastric mucosal changes from normal gastric epithelium to intestinal metaplasia through atrophic gastritis after H. pylori infection using 2-DE and immunoblotting. 2-DE is a useful tool to detect disease-specific proteins and to analyze the detected proteins via mass spectrometry. Auto-antibodies in autoimmune diseases are commonly used in clinical practice as biomarkers, however, novel autoantigens that have been or would be found via 2-D immunoblotting could have a potential to be used in clinical practice as diagnostic biomarkers and therapeutic tools.
Autoimmune diseases associated with H. pylori infection have been reported globally. Even though autoantigens have low or no homogeneity with H. pylori proteins, these studies have clarified that they can induce autoimmune reactions if they have similar conformational epitope with H. pylori. Ko, et al.19 reported that 25 (35.2%) of 71 monoclonal antibodies made by cleaved whole cells of H. pylori cross reacted with gastric epithelial cells in a patient with H. pylori infection, 23 (32.5%) with fetal gastric epithelial cells, 15 (21.1%) with smooth muscle cells, 11 (15.5%) with renal tubular cells, 11 (15.5%) with ductal cells in salivary glands, eight (11.3%) with duodenal epithelial cells, five (7.0%) with inflammatory cells, three (4.2%) with follicular cells in a thyroid gland, and one (1.4%) with colonic epithelial cells. As the aforementioned monoclonal antibodies could react immunologically with fetal gastric epithelial cells that have never been exposed to H. pylori and diverse human cells, H. pylori might harbor antigens capable of cross-reaction with various human tissue antigens. The results suggest that an autoimmune reaction might be involved in the pathogenesis of not only H. pylori-associated gastrointestinal disorders, but also extra-intestinal autoimmune disorders. Autoantigens reported to be involved in diverse autoimmune disorders associated with H. pylori infection include carbonic anhydrase II similar alpha carbonic anhydrase of H. pylori in autoimmune pancreatitis,20 anti-CagA antibody, anti-HSP 65 antibody, and anti-HSP 60 antibody in artherosclerosis,21-23 anti-CagA antibody in Graves disease,24 anti-platelet glycoprotein antibody in immune thrombocytopenic purpura,25 proteins from endothelial or smooth muscle cells against CagA in hypertension,26 and HSP 60 of H. pylori associated with Sjogren syndrome.27 A healthy human body has immune regulatory systems including Treg cells. However, prolonged stimuli to Th1 cells and individual genetic factors can provoke autoimmune reactions.
In 1989, Negrini, et al.28 reported cross reactions between 15 of 21 monoclonal antibodies against H. pylori and different immunohistochemical stains of foveolar cells, pyloric gland, red blood cells, or white blood cells against 21 monoclonal antibodies. They subsequently reported the cross-reaction of gastric epithelial cells with monoclonal antibodies in 84% of 82 patients with H. pylori infection,29 and strong cross-reaction of anti-Lewis monoclonal antibody from the lipopolysaccharide of H. pylori with human and mouse gastric mucosa.30 Uibo, et al.31 also reported the presence of homologous amino acid sequences (72% in 25 amino acid overlap) between H+,K+-ATPase in parietal cells and the urease B subunit of H. pylori. Ma, et al.32 reported that serum auto-antibodies in a patient with type A gastritis could react with H+,K+-ATPase of swine and H. pylori. These reports suggest that anti-H+,K+-ATPase antibodies produced after H. pylori infection could react with parietal cells. However, we could not find H+,K+-ATPase in autoantigenic protein profiles. This might be due to the loss of proton pump proteins during protein extraction process or due to intrinsic problems in our antrectomy specimen in which most parietal cells are intact and most epithelial cells are mucus secreting ones.
We also used AGS cells to discover autoantigens against gastric cells. Although cancer-related antigens are associated with cancer cells, they are also present as autoantigens from normal gastric cells. This was the reason why 2-DE was performed using AGS cells. Because AGS cells are from a cancer cell line, the expected autoantigens were cancer/testis antigen like MAGE-3, differentiation antigen like gp100, tumor specific antigen like mutated p53, mutated p21/ras, overexpressed self antigen like HPV E6/E7, viral antigen like hepatitis C virus, and oncofetal antigen like carcin-embryonic antigen and alpha fetoprotein.33 Clinically, autoantigens in cancer patients are hardly used for diagnosis and severity assessment of their disease, because the autoantigens associated with cancers are also expressed in healthy adults. As an example, one study documented that alpha-enolase or heterogeneous nuclear ribonucleprotein I was expressed in more than 50% of healthy Chinese and annexin II, F-actin capping protein beta subunit and calreticulin were also expressed in more than 20% of healthy Chinese.34 The present detection of alpha-enolase, heat shock 70 kDa protein, annexin II, and preoxiredoxin 6 corroborates the results of the previous study.
We compared the amino acid sequences of the novel gastric mucosal tissue proteins and AGS cells with those of H. pylori to estimate molecular mimicry between human tissue and H. pylori proteins. Homology in amino acid sequences of mitochondrial ATP synthase subunit beta, WD repeat-containing protein 1, mitochondrial 60 kDa heat shock protein, tyrosine-protein phosphatase non-receptor type 11, 78 kDa glucose-regulated protein precursor, heat shock cognate 71 kDa protein, heat shock 70 kDa protein 1, peroxiredoxin-2, alpha-enolase, and glutathione S-transferase P was identified.
If these 10 homologous proteins are similar to the conformational epitope of H. pylori, they might cause gastric mucosal atrophy through an autoimmune mechanism, such as H+,K+-ATPase. However, the amino acid sequence homology differed from the conformational epitope. The approach was limited by the failure to detect H+,K+-ATPase even after three experiments using gastric mucosal tissue. Therefore, we cannot be sure whether our results are restricted to only human gastric mucosal proteins. The present findings implicate gastric lipase and gastrokine 1 as gastric cell-specific autoantigens. Chief cells on fundus secrets gastric lipases, known as acidic lipasese, which have their optimal pH of 5 and control approximately 25% of lipid hydrolysis in adults.35 Further studies are needed to determine whether the autoimmune reaction against gastric lipase is related with the destruction of chief cells on the gastric fundus. Gastrokine 1 is abundant in normal gastric cells, however, it is decreased in H. pylori-infected gastric cells, and the decrease is associated with the delayed recovery of damaged gastric cells.36 Further studies are also warranted as to whether autoimmune reaction against gastrokine 1 is related with the destruction of gastric cells. The absence of homology between gastric lipase, gastrokine 1, and any protein of H. pylori indicates that an autoimmune reaction against gastric lipase and gastrokine 1 might not be directly related to H. pylori infection. However, this speculation is tentative, since the conformational epitopes of the proteins differ from homology of amino acid sequences.
An acknowledged limitation was that our study was an expansive autoantigenic study. Gastric mucosal tissue harbors diversely heterogeneous cells, such as smooth muscle cells, vessels, nerves, and inflammatory cells, besides gastric diverse epithelial cell lines. It is also possible that the results of AGS cells reflect the cancer-specific proteins more importantly presented during carcinogenesis and in cancer cells instead of normal gastric epithelial cell specific antigens. Some autoantbodies against antigenic proteins which we found might have not been involved in H. pylori-derived gastric mucosal alteration, but frequently identified in the healthy population because we used the pooled sera obtained from patients with various disease backgrounds.34 Aforementioned limits should be considered when interpreting our results as autoantigens.
Despite the limitations, Heat shock congnate 70 kDa protein, cytoplasmic actin 1, annexin A3, albumin, alpha-enoase, and leukocyte elastase were detected in both gastric mucosal tissue and AGS cells. Mitochondrial ATP synthase subunit beta, WD repeat-containing protein 1, mitochondrial 60 kDa heat shock protein, tyrosine-protein phosphatase non-receptor type 11, 78 kDa glucose-regulated protein precursor, heat shock cognate 71 kDa protein, heat shock 70 kDa protein 1, peroxiredoxin-2, alpha-enolase, and glutathione S-transferase P showed homology in amino acid sequences with proteins derived from H. pylori.
In summary, we tentatively suggest that newly identified gastric proteins from gastric mucosal tissue and AGS cells might provide tools of control and prevention of gastrointestinal disorders associated with H. pylori infection. These autoantigens might break the vicious cycle in gastroduodenal disorders if their pathophysiological roles in the progress of chronic atrophic gastritis, gastroduodenal ulcers, intestinal metaplasia, and gastric cancer could clearly be understood.
Figures and Tables
 | Fig. 1Autoantigenic proteins visualized by 2-D immunoblotting derived from human gastric mucosa. (A) 2-DE of total protein extracts from human gastric mucosal tissue. (B) 2-D immunoblot of human gastric mucosal tissue by a pool of 300 sera obtained from Helicobacter pylori-seropositive patients. 2-DE, two-dimensional-PAGE. |
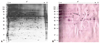 | Fig. 2Autoantigenic proteins visualized by 2-D immunoblotting derived from AGS cells. (A) 2-DE of total protein extracts from AGS cells. (B) 2-D immunoblot of total proteins from AGS cells by a pool of 300 sera obtained from Helicobacter pylori-seropositive patients. 2-DE, two-dimensional-PAGE; AGS, gastric adenocarcinoma cell lines derived from a Caucasian patient who had received no prior therapy. |
 | Fig. 3Common autoantigenic proteins are indicated with red circles on 2-DE silver staining and immunoblotting of gastric mucosal tissue (A) and AGS cells (B). 2-DE, two-dimensional-PAGE. |
Table 1
Mass Spectrometric Identification of Autoantigens of Human Gastric Antrectomy Mucosal Tissue by a Pool of 300 Sera Obtained from Helicobacter Pylori-Seropositive Patients

Table 2
Mass Spectrometric Identification of Autoantigenic Proteins of AGS by a Pool of 300 Sera Obtained from Helicobacter Pylori-Seropositive Patients
ACKNOWLEDGEMENTS
This study was supported by the Multi-year Clinical Research Fund of Gyeongsang National University Hospital in 2008-2009. Biospecimens were provided by the Gyeongsang National University Hospital, which is a member of the National Biobank of Korea supported by the Ministry of Health & Welfare.
References
3. Marshall BJ, Warren JR. Unindentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 1:1311–1315.
4. Goodwin CS, Armstrong JA, Marshall BJ. Campylobacter pyloridis, gastritis, and peptic ulceration. J Clin Pathol. 1986; 39:353–365.

5. Parsonnet J, Vandersteen D, Goates J, Sibley RK, Pritikin J, Chang Y. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst. 1991; 83:640–643.

6. Enno A, O'Rourke JL, Howlett CR, Jack A, Dixon MF, Lee A. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis. A mouse model of Helicobacter pylori-induced gastric lymphoma. Am J Pathol. 1995; 147:217–222.
7. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992; 52:6735–6740.
8. Baik SC, Youn HS, Chung MH, Lee WK, Cho MJ, Ko GH, et al. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 1996; 56:1279–1282.
9. Negrini R, Savio A, Appelmelk BJ. Autoantibodies to gastric mucosa in Helicobacter pylori infection. Helicobacter. 1997; 2:Suppl 1. S13–S16.

10. Kirchner T, Steininger H, Faller G. Immunopathology of Helicobacter pylori gastritis. Digestion. 1997; 58:Suppl 1. 14–16.
11. Youn HS, Ko GH, Chung MH, Lee WK, Cho MJ, Rhee KH. Pathogenesis and prevention of stomach cancer. J Korean Med Sci. 1996; 11:373–385.

12. D'Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004; 10:316–323.
13. Baik SC, Kim JB, Cho MJ, Kim YC, Park CK, Ryou HH, et al. Prevalence of Helicobacter pylori infection among normal Korean adults. J Korean Soc Microbiol. 1990; 25:455–462.
14. Rhee KH, Youn HS, Baik SC, Lee WK, Cho MJ, Choi HJ, et al. Prevalence of Helicobacter pylori Infection in Korea. J Korean Soc Microbiol. 1990; 25:475–490.
15. Faller G, Steininger H, Appelmelk B, Kirchner T. Evidence of novel pathogenic pathways for the formation of antigastric autoantibodies in Helicobacter pylori gastritis. J Clin Pathol. 1998; 51:244–245.

16. Faller G, Kirchner T. Role of antigastric autoantibodies in chronic Helicobacter pylori infection. Microsc Res Tech. 2000; 48:321–326.

17. Heukeshoven J, Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988; 9:28–32.

18. O'Connell KL, Stults JT. Identification of mouse liver proteins on two-dimensional electrophoresis gels by matrix-assisted laser desorption/ionization mass spectrometry of in situ enzymatic digests. Electrophoresis. 1997; 18:349–359.
19. Ko GH, Park HB, Shin MK, Park CK, Lee JH, Youn HS, et al. Monoclonal antibodies against Helicobacter pylori cross-react with human tissue. Helicobacter. 1997; 2:210–215.

20. Guarneri F, Guarneri C, Benvenga S. Helicobacter pylori and autoimmune pancreatitis: role of carbonic anhydrase via molecular mimicry? J Cell Mol Med. 2005; 9:741–744.

21. Birnie DH, Holme ER, McKay IC, Hood S, McColl KE, Hillis WS. Association between antibodies to heat shock protein 65 and coronary atherosclerosis. Possible mechanism of action of Helicobacter pylori and other bacterial infections in increasing cardiovascular risk. Eur Heart J. 1998; 19:387–394.

22. Matsuura E, Kobayashi K, Matsunami Y, Shen L, Quan N, Makarova M, et al. Autoimmunity, infectious immunity, and atherosclerosis. J Clin Immunol. 2009; 29:714–721.

23. Rožanković PB, Huzjan AL, Cupić H, Benčić IJ, Bašić S, Demarin V. Influence of CagA-positive Helicobacter pylori strains on atherosclerotic carotid disease. J Neurol. 2011; 258:753–761.

24. Bassi V, Santinelli C, Iengo A, Romano C. Identification of a correlation between Helicobacter pylori infection and Graves' disease. Helicobacter. 2010; 15:558–562.

25. Asahi A, Nishimoto T, Okazaki Y, Suzuki H, Masaoka T, Kawakami Y, et al. Helicobacter pylori eradication shifts monocyte Fcgamma receptor balance toward inhibitory FcgammaRIIB in immune thrombocytopenic purpura patients. J Clin Invest. 2008; 118:2939–2949.
26. Migneco A, Ojetti V, Specchia L, Franceschi F, Candelli M, Mettimano M, et al. Eradication of Helicobacter pylori infection improves blood pressure values in patients affected by hypertension. Helicobacter. 2003; 8:585–589.

27. Aragona P, Magazzù G, Macchia G, Bartolone S, Di Pasquale G, Vitali C, et al. Presence of antibodies against Helicobacter pylori and its heat-shock protein 60 in the serum of patients with Sjögren's syndrome. J Rheumatol. 1999; 26:1306–1311.
28. Negrini R, Lisato L, Cavazzini L, Maini P, Gullini S, Basso O, et al. Monoclonal antibodies for specific immunoperoxidase detection of Campylobacter pylori. Gastroenterology. 1989; 96(2 Pt 1):414–420.

29. Negrini R, Lisato L, Zanella I, Cavazzini L, Gullini S, Villanacci V, et al. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991; 101:437–445.

30. Appelmelk BJ, Simoons-Smit I, Negrini R, Moran AP, Aspinall GO, Forte JG, et al. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996; 64:2031–2040.

31. Uibo R, Vorobjova T, Metsküla K, Kisand K, Wadström T, Kivik T. Association of Helicobacter pylori and gastric autoimmunity: a population-based study. FEMS Immunol Med Microbiol. 1995; 11:65–68.

32. Ma JY, Borch K, Sjöstrand SE, Janzon L, Mårdh S. Positive correlation between H,K-adenosine triphosphatase autoantibodies and Helicobacter pylori antibodies in patients with pernicious anemia. Scand J Gastroenterol. 1994; 29:961–965.

33. Dermime S, Gilham DE, Shaw DM, Davidson EJ, Meziane EK, Armstrong A, et al. Vaccine and antibody-directed T cell tumour immunotherapy. Biochim Biophys Acta. 2004; 1704:11–35.

34. Li WH, Zhao J, Li HY, Liu H, Li AL, Wang HX, et al. Proteomics-based identification of autoantibodies in the sera of healthy Chinese individuals from Beijing. Proteomics. 2006; 6:4781–4789.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download