Abstract
Purpose
Although some CDH13 single nucleotide polymorphisms (SNPs) have been shown to be determinants of blood adiponectin levels, the clinical implications of CDH13 variants are not yet completely understood. The purpose of this study was to evaluate the effects of SNPs of CDH13 on metabolic and vascular phenotypes.
Materials and Methods
We included 238 hypertensive subjects and 260 age- and sex-matched controls. Seven tagging-SNPs were identified in the CDH13 gene by whole gene sequencing. The association between these SNP variants and the risk of hypertension, metabolic traits, and carotid intima-media thickness (IMT) was examined.
Results
Minor allele carriers of rs12444338 had a lower risk of hypertension, but the association turned out just marginal after adjusting confoudners. Blood glucose levels were higher in the minor allele carriers of c.1407C>T (p=0.01), whereas low-density lipoprotein-cholesterol levels were greater in those of rs6565105 (p=0.02). The minor allele of rs1048612 was associated with a higher body mass index (p=0.01). In addition, the mean carotid IMT was significantly associated with rs12444338 (p=0.02) and rs1048612 (p=0.02).
CDH13 has been reported to encode T-cadherin, an adiponectin receptor discovered after adipoR1 and adipoR2.1 It is characteristically expressed on vascular cells such as endothelial cells and smooth muscle cells.2 Prior experimental studies suggested that T-cadherin modulates tissue and circulating adiponectin levels, therefore affecting vascular remodeling, inflammation, and atherosclerosis.1,3,4 However, the role of T-cadherin in adiponectin signaling into the cells and its clinical implication is not completely understood.
Asian people exhibit the characteristic phenotype of adiposity5 and adiposity-related lipid6 or vascular traits.5 However, studies on genetic basis of these relationships have been insufficient in this population. To date, several CDH13 single nucleotide polymorphisms (SNPs) including rs3865188 have been shown to be determinants of blood adiponectin levels in Asians.7-9 The association between rs11646213, a CDH13 SNP, and blood pressure phenotype has also been reported in European cohorts.10 Chung, et al.11 demonstrated that rs4783244, another SNP, is associated with the metabolic syndrome phenotype in Taiwanese. Although they provided possible evidence of a relationship with CDH13-cardiometabolic disease, the association between the variant and hypertension was not clear.
Here we examined the effect of seven SNPs of the CDH13 gene on cardiometabolic traits in a cohort of 498 Koreans. The purpose of the study was to evaluate the association between the seven SNPs and hypertension risk, metabolic traits, and carotid atherosclerosis. In the current study, we found an associations between rs12444338 and hypertension, which was marginal, and carotid intima-media thickness (IMT). Another CDH13 SNP, rs1048612, was found to be related to body mass index and carotid IMT. We also found two more SNPs that are associated with blood glucose and cholesterol levels. Our study shows the multifaceted effect of CDH13 variants on metabolic and vascular traits.
Two hundred thirty-eight unrelated Korean subjects with hypertension and 260 age- and sex-matched healthy control subjects were recruited for this study. Subjects were drawn from the Cardiovascular Genome Center Registry from Yonsei University Health System. Briefly, consecutive subjects who visited Severance Cardiovascular Hospital in Seoul, Korea were prospectively screened for hypertension using several inclusion criteria. Subjects who had systolic blood pressure (BP) ≥135 mm Hg and/or diastolic BP ≥85 mm Hg during three different visits before taking antihypertensive medications or who were already taking such medications were recruited. Hypertensive subjects who were diagnosed at 30-60 years of age were eligible for the study. Two hundred sixty unrelated Koreans without a diagnosis of hypertension were recruited from Cardiology Clinic in the same hospital. Control subjects had to have a systolic BP <135 mm Hg and diastolic BP <85 mm Hg without taking BP-lowering agents. Exclusion criteria were structural heart disease, coronary artery disease, secondary hypertension, diabetes mellitus, or serum creatinine >1.4 mg/dL. This study was conducted under the approval of the local Institutional Review Board.
On the day of enrollment, clinical data including demographic variables and medical history were recorded. Venous blood samples were collected after an overnight fast and samples were analyzed within 4 hours of collection. All analyses were conducted by a local laboratory, certified by the Korean Society of Laboratory Medicine.
Carotid IMT was measured as a surrogate marker of atherosclerosis. This examination was only available for study subjects enrolled in the registry in the last 3 years. A longitudinal B-mode image of common carotid arteries was obtained by 8-MHz linear scanner (Sequoia C512, Acuson Co Ltd., Oceanside, CA, USA) and stored digitally. The mean carotid IMT of the far walls of the common carotid arteries were measured 1 cm proximal to the carotid bifurcation on the end-diastolic frame using an automated edge-detection method program (M'ATH, METRIS Co., Argenteuil, France).
PCR primers were designed to independently amplify CDH13 fragments. Primer sequences are available on request. PCR products were purified and then sequenced using a BigDye Terminator Cycle Sequencing Kit (version 3.1, ABI, Foster City, CA, USA) and ABI 3730×1 automated sequencer (Applied Biosystems, Foster City, CA, USA). The sequencing primers were the same as those used for PCR amplification. Mutation analyses were conducted using Phred, Pharp, Consed, Polyphred 5.04 software (http://droog.mbt.washington.edu/PolyPhred.html).
Seven tagging-SNPs identified in the CDH13 gene by whole gene sequencing were genotyped. Genomic DNA was extracted from 5 mL of peripheral venous blood using a commercially available isolation kit (QuickGene SP Kit DNA whole blood, Fujifilm, Tokyo, Japan). Genotyping was performed using the TaqMan fluorogenic 5' nuclease assay (ABI).
Group differences for categorical variables were assessed by chi-square test and continuous variables were examined by Student's t-test. The association between genotype and hypertension was evaluated using odds ratios (ORs) and 95% confidence intervals (CIs) from chi-square tests and logistic regression analyses. Possible genotype-related differences in other cardiometabolic traits such as body mass index, blood glucose, triglyceride, low-density lipoprotein-cholesterol (LDL-C), and carotid IMT values were analyzed by Kruskal-Wallis test. The recessive model tested the association of having one homozygous risk allele (1) vs. having at least one non-risk allele in homozygous (0) or in heterozygous (0). The additive model tested the association that depend additively upon the risk or minor allele, 0 for homozygous non-risk alleles, 1 for heterozygous alleles and 2 for homozygous risk alleles. We estimated our power to detect the association. For minor allele frequencies of 0.3-0.4, our study had 80% power to detect odds ratios as small as 1.44-1.46. In these analyses, 33-38 outliers by standard quartile method were excluded. Results are expressed as mean±SD or median (interquartile range) and p values were computed from each model of association. All data were analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Characteristics of the case-control population are shown in Table 1. The median age was 51 years and females made up 50% of the population. Cases had higher body mass index, blood glucose, and triglyceride levels, but lower high-density lipoprotein-cholesterol levels. The seven tagging-SNPs were rs6565051, rs7204454, c.1407C>T, rs12444338, rs72807847, rs6565105, and rs1048612. The minor allele frequencies were 0.027-0.394 for each SNP (Table 2).
One SNP, rs12444338, was significantly associated with hypertension. In the first additive model, that adjusted for age and sex, the TT genotype of SNP was associated with a decreased risk of hypertension [OR=0.74 (CI 0.56-0.98), p=0.03] (Table 2). However, in model 2 after further adjustment for metabolic variables, the association was only marginally significant. No other associations were observed between the genotypes of other SNPs and hypertension risk.
In further examinations of the variants with other metabolic traits, blood glucose levels increased with the number of C alleles in c.1407C>T SNP (p=0.01). In addition, the AA genotype of rs6565105 showed higher LDL-C levels (p=0.02), whereas the AA genotype of rs1048612 was associated with higher body mass index (p=0.01) (Table 3).
The mean carotid IMT decreased with the number of T allele of rs12444338 in the additive model (p=0.02). The AA genotype of rs1048612 was associated with greater IMT, compared to the GG or GA genotype of the SNP (p=0.02) (Fig. 1, Supplementary Table 1).
Haplotypes of SNPs of rs6565051-rs7204454 and those of rs6565051-rs7204454-C_1407T-rs12444338-rs72807847-rs6565105 were evaluated. Two combinations were associated with body mass index, another one with glucose levels, and another one with carotid IMT (Supplementary Table 2). These findings further provide potential additive effects of SNPs of CDH13, although exploration of the corresponding mechanism was beyond the purpose of our study.
The current study demonstrates no significant association between SNP located within CDH13 and hypertension in Koreans. Only marginal association was observed between rs12444338 and hypertension. We demonstrated that a minor allele of this SNP imparted a thinner carotid IMT. In addition, rs1048612, which showed an association with body mass index, also had an effect on carotid IMT. Other CDH13 SNPs, such as c.1407C>T and rs6565105, were associated with metabolic traits including blood glucose and cholesterol levels, respectively. Our study shows the multifaceted effects of CDH13 variants on metabolic and vascular traits.
Recently, an association between a SNP of CDH13 and blood pressure was identified and replicated in Europeans. In that report, carriers of the minor allele of rs11646213 had a decreased risk of hypertension.10 In another ethnic group, CDH13 variants also had an effect on blood pressure, but top SNPs were different from the prior study.12 In our study, rs12444338 variants demonstrated a marginal association with hypertension risk. This SNP is located in the promoter region of CDH13.7,13 It is not clear how rs12444338 affects the blood pressure phenotype; however, the association of rs12444338 polymorphisms and the changed promoter activity of CDH13 may suggest this variant can play a role in CDH13 regulation and phenotypic change.7
We found associations between two SNPs, rs12444338 and rs1048612 and carotid IMT. The effect of rs12444338 was significant on additive and dominant models and seems to be influenced by heterozygote subjects. However, the association was not obvious in the recessive model; therefore, further confirmation in a larger population is needed. Although it was previously reported that another SNP of CDH13, rs4783244, is associated with carotid IMT and stroke risk,11 this is the first time that these two SNPs have shown an effect on vascular phenotype. Traditional cardiovascular risk factors, including age, sex, hypertension, smoking, hypercholesterolemia, and body mass index, have been reported as determinants of carotid IMT.14,15 The effect of rs12444338 on blood pressure and that of rs1048612 on body mass index were identified in our results, and these findings are concordant to the association of the two SNP and carotid IMT. CDH13-encoded T-cadherin, when ligated, can activate nuclear factor-κB signaling and promote inflammation.16 Furthermore, T-cadherin expression is associated with vascular smooth muscle cell proliferation.4 These biological functions may be possible processes linking CDH13 variant and carotid atherosclerosis. However, CDH13 has been ranked as having the highest number of interaction partners17 and the mechanism of the effects of CDH13 variants on carotid atherosclerosis may be more complex than we now estimate.
In our study, carriers of a minor allele of c.1407C>T had higher glucose levels, whereas those of rs6565105 had higher LDL-C levels. This is the first report to uncover an association between these two SNPs and metabolic traits. As previously mentioned, the CDH13 gene is a strong determinant of blood adiponectin level, and this level is correlated with various metabolic traits including glucose levels, insulin levels, and dyslipidemia.18 Therefore, the association between the c.1407C>T variant and glucose may be partly mediated by an adiponectin-related mechanism. The rs4783244 genotype of CDH13 was found to be correlated to glucose levels, as well as triglyceride levels or abdominal obesity, in Asians.11 Similarly, in a Swedish cohort, Fava, et al.19 demonstrated that the CDH13 rs11645213 T>A polymorphism is associated with metabolic syndrome. On the other hand, T-cadherin, coded by CDH13, was reported to modulate signaling and cellular response to insulin;20 this report is also in accordance with our results. The relationship between CDH13 variants and cholesterol level has not been well established. However, Dong, et al.21 recently demonstrated that two SNPs of CDH13, rs4357934 and rs1164264, were significantly associated with total and LDL-C levels. This may be possible evidence that supports our data.
The strength of our study include that we systematically evaluated uncovered various effects of CDH13 variants on metabolic and vascular phenotypes. For instance, minor allele carriers of rs1048612 had a higher body mass index and carotid IMT. Although the cause-effect relationship is unclear between the variants and the phenotypes at this stage, our findings may provoke further study on the clinical implications of this gene. Furthermore, we confirmed the vascular effect of rs12444338 in a Korean population. Previously this SNP was shown to have an association with blood adiponectin levels.7 Although our data do not include adiponectin concentrations, the current study is the first to report the effect of rs12444338 on cardiometabolic phenotype.
The present study has several limitations. First, although we demonstrated the effects of SNPs of CDH13 on cardiometabolic phenotype, their significance was only modest. In other association studies, the optimal method among different genetic models is not clear. When we tested a single genetic model that matches the actual underlying mode of inheritance of the causal allele, we were able to achieve maximal power. However, when the inheritance pattern of the causal allele is unknown, analyzing using multiple genetic models together is more powerful. In this study, we analyzed our data using multiple models together to investigate the association signals to phenotype. The association signals can be significant in only one genetic model when the sample size is small. As a next step, replication studies may be helpful to derive a clearer conclusion on the variants' effects. Second, as previously mentioned, we did not measure blood adiponectin or high molecular weight adiponectin levels in our subjects. We are not sure if those levels are be correlated with the studied variants or evaluated phenotypes. However, adiponectin values may be a good candidate to explain the link between the studied variants and cardiometabolic phenotypes. Finally, we assessed carotid IMT in a group of our subjects, and certain analyses on the variants and IMT might not have obtained statistical significance.
In conclusion, our study provides evidence that CDH13 variants may be associated with metabolic traits and carotid atherosclerosis in Koreans. Our study shows the multifaceted effects of CDH13 variants on metabolic and vascular risk.
Figures and Tables
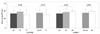 | Fig. 1Bar graph showing carotid IMT in each genotype of rs12444338 and rs1048612. IMT, intima-media thickness. |
Table 2
Associations between Genotypes of the Studied Variants and Hypertension
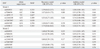
MAF, minor allele frequency; OR, odds ratio; SNP, single nucleotide polymorphism; CI, confidence interval; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol.
The p value and ORs were calculated with multivariate logistic regression analysis with adjustment for age, sex (model 1), or age, sex, body mass index, waist circumference, glucose, triglyceride, HDL-C, and LDL-C (model 2).
*p<0.05.
ACKNOWLEDGEMENTS
This research was financially supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2012R1A1A2039828), MEST through the National Research Foundation of Korea (Grant No. 2012R1A4A1029061), and the Creative Allied Project (CAP) grant funded by the Korean Research Council of Fundamental Science and Technology (KRCF).
References
1. Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004; 101:10308–10313.

2. Takeuchi T, Adachi Y, Ohtsuki Y, Furihata M. Adiponectin receptors, with special focus on the role of the third receptor, T-cadherin, in vascular disease. Med Mol Morphol. 2007; 40:115–120.

3. Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000; 102:1296–1301.

4. Kudrjashova E, Bashtrikov P, Bochkov V, Parfyonova Y, Tkachuk V, Antropova J, et al. Expression of adhesion molecule T-cadherin is increased during neointima formation in experimental restenosis. Histochem Cell Biol. 2002; 118:281–290.

5. Lee YH, Lee SH, Jung ES, Kim JS, Shim CY, Ko YG, et al. Visceral adiposity and the severity of coronary artery disease in middle-aged subjects with normal waist circumference and its relation with lipocalin-2 and MCP-1. Atherosclerosis. 2010; 213:592–597.

6. Choi SJ, Park SH, Lee KS, Park HY. The prevalence, awareness and treatment of high low density lipoprotein-cholesterol in korean adults without coronary heart diseases - the third Korea national health and nutrition examination survey, 2005 -. Korean Circ J. 2012; 42:86–94.

7. Jee SH, Sull JW, Lee JE, Shin C, Park J, Kimm H, et al. Adiponectin concentrations: a genome-wide association study. Am J Hum Genet. 2010; 87:545–552.

8. Wu Y, Li Y, Lange EM, Croteau-Chonka DC, Kuzawa CW, McDade TW, et al. Genome-wide association study for adiponectin levels in Filipino women identifies CDH13 and a novel uncommon haplotype at KNG1-ADIPOQ. Hum Mol Genet. 2010; 19:4955–4964.

9. Morisaki H, Yamanaka I, Iwai N, Miyamoto Y, Kokubo Y, Okamura T, et al. CDH13 gene coding T-cadherin influences variations in plasma adiponectin levels in the Japanese population. Hum Mutat. 2012; 33:402–410.

10. Org E, Eyheramendy S, Juhanson P, Gieger C, Lichtner P, Klopp N, et al. Genome-wide scan identifies CDH13 as a novel susceptibility locus contributing to blood pressure determination in two European populations. Hum Mol Genet. 2009; 18:2288–2296.

11. Chung CM, Lin TH, Chen JW, Leu HB, Yang HC, Ho HY, et al. A genome-wide association study reveals a quantitative trait locus of adiponectin on CDH13 that predicts cardiometabolic outcomes. Diabetes. 2011; 60:2417–2423.

12. Adeyemo A, Gerry N, Chen G, Herbert A, Doumatey A, Huang H, et al. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet. 2009; 5:e1000564.

14. Cardoso CR, Marques CE, Leite NC, Salles GF. Factors associated with carotid intima-media thickness and carotid plaques in type 2 diabetic patients. J Hypertens. 2012; 30:940–947.

15. Su TC, Chien KL, Jeng JS, Chen MF, Hsu HC, Torng PL, et al. Age- and gender-associated determinants of carotid intima-media thickness: a community-based study. J Atheroscler Thromb. 2012; 19:872–880.

16. Kipmen-Korgun D, Osibow K, Zoratti C, Schraml E, Greilberger J, Kostner GM, et al. T-cadherin mediates low-density lipoprotein-initiated cell proliferation via the Ca(2+)-tyrosine kinase-Erk1/2 pathway. J Cardiovasc Pharmacol. 2005; 45:418–430.

17. de las Fuentes L, Yang W, Dávila-Román VG, Gu C. Pathway-based genome-wide association analysis of coronary heart disease identifies biologically important gene sets. Eur J Hum Genet. 2012; 20:1168–1173.

18. Díez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003; 148:293–300.
19. Fava C, Danese E, Montagnana M, Sjögren M, Almgren P, Guidi GC, et al. A variant upstream of the CDH13 adiponectin receptor gene and metabolic syndrome in Swedes. Am J Cardiol. 2011; 108:1432–1437.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download