Abstract
Purpose
Although there is no clinical evidence of nephrotoxicity with the volatile anesthetics currently used in general anesthesia, a better agent should be needed in terms of preserving postoperative renal function in living kidney donors who have only single remaining kidney. The purpose of the current retrospective, single-center study was to evaluate and compare renal function of living kidney donors after nephrectomy under either sevoflurane or desflurane anesthesia.
Materials and Methods
From January 2006 through December 2011, a total of 228 donors undergoing video assisted minilaparotomy surgery nephrectomy for kidney donation were retrospectively enrolled in the current study. The donors were categorized into a sevoflurane group or desflurane group based on the type of volatile anesthetic used. We collected laboratory data from the patients preoperatively, immediately after the operation, on the first postoperative day and on the third postoperative day. We also compared renal function of the kidney donors after donor nephrectomy by comparing creatinine level and estimated glomerular filtration rate (eGFR).
The nobility of donors and the belief that donation will not harm the donor have increased the frequency of kidney donation from living donors.1 Although the existing body of evidence suggests that living kidney donors have medical outcomes similar to those in the general population,2 several reports have demonstrated the potential risks of developing hypertension, proteinuria, and end stage renal disease (ESRD).1,3 In addition, many institutes have expanded the selection criteria for donors, accepting donors with well-controlled hypertension and advanced age due to increasing number of patients reaching ESRD and the improvement in clinical outcomes of renal allograft.4 For these reasons, all efforts should be concentrated on ensuring the safety of patients and preserving the function of the remaining kidney during anesthesia maintenance. However, most studies have focused on the anesthesia and intraoperative management of the recipients rather those of the donors.5-8 Therefore, the adequacy of current anesthetic management for donors should be evaluated as well.
Volatile anesthetics have been frequently used for general anesthesia for nephrectomy of kidney donor.9 The typical volatile anesthetics commonly used nowadays are sevoflurane and desflurane.10-13 Sevoflurane has the potential to adversely affect the kidney function because an inorganic fluoride ion from the defluorination of sevoflurane and compound A from reaction with carbon dioxide absorbent are associated with nephrotoxicity.14,15 On the other hand, desflurane is extremely resistant to defluorination, and it does not appear to be nephrotoxic.16 Although any nephrotoxic effect of sevoflurane in human has not yet been proven, this issue is still subject to debate due to many literatures related to sevoflurane induced nephrotoxicity.14,15,17-19 Recently, a study performed in living donor hepatectomy demonstrated better postoperative kidney function with desflurane than with sevoflurane.20
Although there is no clinical evidence of nephrotoxicity with the volatile anesthetics currently used in general anesthesia, a better agent should be chosen in terms of preserving postoperative kidney function in living donors who have only a single kidney remaining. The purpose of the current retrospective, single-center study was to evaluate and compare kidney function of living donors after nephrectomy under either sevoflurane or desflurane anesthesia.
From January 2006 through December 2011, a total of 228 donors undergoing nephrectomy for kidney donation under sevoflurane or desflurane anesthesia were retrospectively enrolled in the current study. Patients undergoing perioperative transfusion or re-operation were excluded. All the donors underwent preoperative evaluation including a complete history, physical examination, and laboratory assessment to rule out diseases of major organs, infections, and other systemic illness. Abdominal-pelvic computed tomography with angiography was performed to investigate the anatomy of the kidney and vascular structures before surgery.
The nephrectomies were performed by two urologists using video-assisted minilaparotomy surgery (VAMS).21 The patients were managed intraoperatively using the standard anesthesia protocol for our institution.
Upon arrival at the operating room, the donors were monitored with pulse oximetry, noninvasive arterial blood pressure (BP), electrocardiography, and capnography. Anesthesia was induced with either intravenous propofol (2 mg/kg) or thiopental (3-5 mg/kg) and a continuous infusion of remifentanil (0.1-0.15 µg·kg-1·min-1). Rocuronium (0.6 mg/kg) was given to achieve adequate muscle relaxation before endotracheal intubation. After endotracheal intubation, an additional intravenous catheter was inserted into the external jugular vein or antecubital vein. For anesthesia maintenance, the volatile anesthetic chosen by the attending anesthesiologists was carefully titrated to maintain an end-tidal concentration of 1-1.5 minimal alveolar concentration (MAC) with 50% oxygen in air mixture. Continuous infusion of remifentanil was adjusted to maintain intraoperative BP and heart rate within 20% of the preoperative values. Arterial hypotension during anesthesia maintenance was treated by adjustment of anesthesia level and fluid therapy instead of using vasopressor. The amount of administered fluid was initially 10 mL/kg/hour and was adjusted to maintain an adequate urine output of greater than 100 mL/hour. Mannitol (0.5 g/kg) was routinely administered before manipulation of the kidney. Inadequate urine output was treated with 300-500 mL of loading fluid or intravenous administration of 5-10 mg furosemide when necessary. Heparin (70 unit/kg) was given intravenously before vessel ligation. After removal of the kidney, the amount of administered fluid was maintained minimally, and protamine sulfate (0.7 mg/kg) was given intravenously. At the end of the operation, the inhaled anesthetic was discontinued, and the neuromuscular block was reversed with intravenous administration of 0.2 mg glycopyrrolate and 1 mg neostigmine. After endotracheal extubation, the donors were transferred to the post anesthesia care unit (PACU). For postoperative pain control, intravenous patient-controlled analgesia, using fentanyl without non-steroidal anti-inflammatory drug, was administered.
Medical records were reviewed and laboratory data were collected for investigation. The donors enrolled in the current study were assigned to either the desflurane or sevoflurane group based on the volatile anesthetic used.
Patient characteristics including gender, age at operation, height, weight, body mass index, and medical history were recorded. Intraoperative data included surgical and anesthetic times, administered fluids, intraoperative blood loss, urine output, intraoperative transfusion of blood products, and use of furosemide. Duration of stay in the PACU, duration of postoperative hospital stay, and postoperative transfusion of blood products were assessed as postoperative data. We collected laboratory data from the patients preoperatively, immediately after the operation, on the first postoperative day, and on the third postoperative day. The laboratory data included hemoglobin, hematocrit, platelet count, prothrombin time, albumin, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, creatinine (Cr), and estimated glomerular filtration rate (eGFR). Estimated GFR was calculated using the Modification of Diet in Renal Disease formula with age, gender, race, and serum creatinine as variables.22 The following variables were used to compare kidney function before and after surgery and were calculated from the data obtained: ΔCreatinine (value of postoperative Cr-value of preoperative Cr), ΔeGFR (value of postoperative eGFR-value of preoperative eGFR), ΔCreatinine% (ΔCreatinine/value of preoperative Cr), and ΔeGFR% (ΔeGFR/value of preoperative eGFR).
We analyzed the data with SPSS version 18 (SPSS Inc., Chicago, IL, USA). Continuous data were presented as mean (standard deviation) and were analyzed using the independent Student t-test or Mann-Whitney U test. Categorical data were presented as numbers (percentages) and were analyzed using the chi-square test or Fisher's exact test. p<0.05 was considered statistically significant.
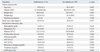
Among the 228 donors enrolled in the study, six donors (one from the desflurane group and five from the sevoflurane group) were excluded because of red blood cell transfusions during the perioperative period. As a result, we ultimately investigated 113 donors in the desflurane group and 109 donors in the sevoflurane group. There were no differences in donor characteristics and preoperative laboratory data between the two groups (Table 1).
Table 2 shows the intraoperative and postoperative data of both groups. The surgical time of the desflurane group was significantly shorter than that of the sevoflurane group (p=0.019), but there was no significant difference in anesthetic time between the two groups (p=0.163). Duration of PACU stay of the desflurane group was significantly shorter than that of the sevoflurane group (55.6±21.9 min vs. 68.0±30.2 min, p=0.001). However, postoperative hospital stay did not differ between the two groups.
Figs. 1 and 2 compare the results of postoperative kidney function tests between the two groups. The decrease in kidney function after surgery was the most prominent on the first postoperative day for both groups. There were no significant differences in the postoperative changes in creatinine and eGFR between the two groups.
In this study, we compared the postoperative kidney function of living donors according to the type of volatile anesthetic used during nephrectomy. The similar outcomes observed between the sevoflurane group and the desflurane group suggest that the choice of volatile anesthetic did not affect residual kidney function after donor nephrectomy.
Sevoflurane has many advantages such as pleasant odor, no pungency, and bronchodilating effect, while desflurane does not have these qualities.10 However, the concern about the nephrotoxicity of sevoflurane exists still.19 The issues of nephrotoxicity related to sevoflurane use are based not only on its fluoride metabolite but also on compound A.10,23 Early reports of fluoride-associated nephrotoxicity from the metabolism of volatile anesthetics focused on methoxyflurane and enflurane, and the toxic threshold of inorganic fluoride associated with nephrotoxicity was found to be 50 µmol/L.24,25 Previous investigations of sevoflurane metabolism demonstrated that a fluoride ion concentration greater than 50 µmol/L could be observed even though sevoflurane was used during operations of average duration. Still, no renal toxicity was demonstrated.14,26 Compound A is the other concerned metabolite associated with the use of sevoflurane. Compound A, or fluoromethyl-2-2-difluoro-1-(trifluoromethyl) vinyl ether, is formed during the interaction of sevoflurane with carbon dioxide absorbents, and it has been shown to be a dose-dependent nephrotoxin in rats.15 In contrast, desflurane does not appear to be nephrotoxic because of its resistance to defluorination and no increase in serum fluoride concentration after exposure to desflurane.10
Previous findings in comparison of kidney function between patients receiving sevoflurane or desflurane were not consistent. A study which compared the effects of sevoflurane and desflurane on kidney function after living donor hepatectomy demonstrated better postoperative kidney function with desflurane than with sevoflurane.20 However, another study on postoperative renal responses following the use of desflurane, sevoflurane, or propofol reported that changes in postoperative kidney function were not affected by the choice of anesthetic.18 Many conditions including the extent of surgical stress, surgical site, preoperative kidney function, and intraoperative hemodynamics can affect postoperative kidney function.18,20 Differences in these conditions could potentially explain for the inconsistent results of studies looking at postoperative kidney function. The safety of sevoflurane with regard to kidney function in this study may be the result of a rapid decline in plasma fluoride concentration due to its lower availability because of a faster washout.19 Also, the site of metabolism is an important factor in fluoride-induced toxicity. That is, intrarenal metabolism of inhaled anesthetics contributes to nephrotoxic effects. Intrarenal metabolism of methoxyflurane and subsequent intrarenal production of fluoride ion are considered to be a significant cause of the nephrotoxic effects of methoxyflurane. The possibility of nephrotoxicity with sevoflurane is counterbalance by its minimal intrarenal metabolism.27 Furthermore, several studies, especially a randomized study in patients with pre-existing renal disease, have failed to demonstrate the existence of nephrotoxic effects associated with compound A in humans.28,29
The difference in surgical technique for living donor nephrectomy can affect postoperative kidney function. A previous study showed that the decline in kidney function in laparoscopic donor nephrectomy was significantly greater than that in open donor nephrectomy because of pneumoperitoneum and prolonged anesthesia use.30 The VAMS approach is a safe and minimally invasive technique for donor nephrectomy, with favorable outcomes including less postoperative pain and a quick recovery. It has been performed over the years in our institute.21 Therefore, the impact of surgical technique on postoperative outcome in this study was thought to be negligible.
Intraoperative data from this study showed that the sevoflurane group had a longer surgical time, whereas the anesthetic times of both groups were similar. The nephrotoxicity of sevoflurane is closely correlated with the duration of its exposure in terms of fluoride and compound A.25,31 In this study, the duration of exposure of volatile anesthetics was similar between the two groups, given the lack of difference in anesthetic times. The variables related to postoperative kidney function of the sevoflurane group were comparable to those of the desflurane group.
This study has several limitations. First, since the design of this study was retrospective, an additional prospective study is required. In this study, surgical technique and anesthesia protocol were applied equally to all of the donors, and there were a sufficient number of donors enrolled. In prospective studies of donors, a higher level of ethics and safety are required. Thus, sufficient evidence should be obtained through companion papers and retrospective studies before performing a prospective study. Second, we investigated only traditional parameters of kidney function. Biomarkers such as neutrophil gelatinase-associated lipocalin, cystatin C, and interleukin-18 have been proposed for early detection of acute kidney injury.32 Because of widely varying diagnostic characteristics reported from previous studies and the necessary identification of significant factors that may confuse biomarker interpretation in the perioperative period, these biomarkers are not yet applicable for use in routine clinical practice.33 Third, the dose of sevoflurane and desflurane used for anesthesia maintenance might not be equipotent because the end-tidal concentration during anesthesia could not be maintained with identical MAC values in both groups.
The results of this study revealed comparable postoperative kidney function with sevoflurane or desflurane anesthesia use in living donors undergoing VAMS nephrectomy. It is concluded that sevoflurane and desflurane can be used safely as volatile anesthetics in donors undergoing nephrectomy.
Figures and Tables
 | Fig. 1Comparisons of ΔCreatinine and ΔCreatinine% between the desflurane group and sevoflurane group. The box contains the middle 50% of the data, and the line in the box indicates the median value of the data. The upper edge of the box represents the 75th percentile of the data set, and the lower edge represents the 25th percentile. The range of the middle two quartiles means the inter-quartile range. The ends of the vertical lines represent the minimum and maximum values of the data set unless outliers do not exist, in which case the vertical lines extend to a maximum of 1.5 times the inter-quartile range. Any data that does not exist between the vertical lines should be marked as an outlier with a circle. ΔCreatinine, value of postoperative creatinine-value of preoperative creatinine; ΔCreatinine%, ΔCreatinine/value of preoperative creatinine test; A, value immediately after operation-preoperative value; B, value on the first postoperative day-preoperative value; C, value on the third postoperative day-preoperative value. |
 | Fig. 2Comparisons of ΔeGFR and ΔeGFR% between the desflurane group and sevoflurane group. The box contains the middle 50% of the data, and the line in the box indicates the median value of the data. The upper edge of the box represents the 75th percentile of the data set, and the lower edge represents the 25th percentile. The range of the middle two quartiles means the inter-quartile range. The ends of the vertical lines represent the minimum and maximum values of the data set unless outliers do not exist, in which case the vertical lines extend to a maximum of 1.5 times the inter-quartile range. Any data that does not exist between the vertical lines should be marked as an outlier with a circle. ΔeGFR, value of postoperative estimated glomerular filtration rate-value of preoperative estimated glomerular filtration rate; ΔeGFR%, eGFR/value of preoperative estimated glomerular filtration rate; A, value immediately after operation-preoperative value; B, value on the first postoperative day-preoperative value; C, value on the third postoperative day-preoperative value. |
References
1. Ommen ES, Winston JA, Murphy B. Medical risks in living kidney donors: absence of proof is not proof of absence. Clin J Am Soc Nephrol. 2006; 1:885–895.

2. Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, et al. Long-term consequences of kidney donation. N Engl J Med. 2009; 360:459–469.

3. Gossmann J, Wilhelm A, Kachel HG, Jordan J, Sann U, Geiger H, et al. Long-term consequences of live kidney donation follow-up in 93% of living kidney donors in a single transplant center. Am J Transplant. 2005; 5:2417–2424.

4. Tent H, Sanders JS, Rook M, Hofker HS, Ploeg RJ, Navis G, et al. Effects of preexistent hypertension on blood pressure and residual renal function after donor nephrectomy. Transplantation. 2012; 93:412–417.

5. Modesti C, Sacco T, Morelli G, Bocci MG, Ciocchetti P, Vitale F, et al. Balanced anestesia versus total intravenous anestesia for kidney transplantation. Minerva Anestesiol. 2006; 72:627–635.
6. Teixeira S, Costa G, Costa F, da Silva Viana J, Mota A. Sevoflurane versus isoflurane: does it matter in renal transplantation? Transplant Proc. 2007; 39:2486–2488.

7. Shah VR, Butala BP, Parikh GP, Vora KS, Parikh BK, Modi MP, et al. Combined epidural and general anesthesia for paediatric renal transplantation-a single center experience. Transplant Proc. 2008; 40:3451–3454.

8. Bhosale G, Shah V. Combined spinal-epidural anesthesia for renal transplantation. Transplant Proc. 2008; 40:1122–1124.

9. Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003; 348:2110–2124.

10. Young CJ, Apfelbaum JL. Inhalational anesthetics: desflurane and sevoflurane. J Clin Anesth. 1995; 7:564–577.

11. Kim JM, Lee JH, Lee HJ, Koo BN. Comparison of emergence time in children undergoing minor surgery according to anesthetic: desflurane and sevoflurane. Yonsei Med J. 2013; 54:732–738.

12. Kim ES, Chang HW. The effects of a single bolus of remifentanil on corrected QT interval change during sevoflurane induction. Yonsei Med J. 2011; 52:333–338.

13. Chang DJ, Choi SH, Choi YS, Min KT. Effect of charcoal filter on the emergence from sevoflurane anesthesia in a semi-closed rebreathing circuit. Yonsei Med J. 2011; 52:668–672.

14. Kobayashi Y, Ochiai R, Takeda J, Sekiguchi H, Fukushima K. Serum and urinary inorganic fluoride concentrations after prolonged inhalation of sevoflurane in humans. Anesth Analg. 1992; 74:753–757.

15. Keller KA, Callan C, Prokocimer P, Delgado-Herrera L, Friedman MB, Hoffman GM, et al. Inhalation toxicity study of a haloalkene degradant of sevoflurane, Compound A (PIFE), in Sprague-Dawley rats. Anesthesiology. 1995; 83:1220–1232.

16. Smiley RM, Ornstein E, Pantuck EJ, Pantuck CB, Matteo RS. Metabolism of desflurane and isoflurane to fluoride ion in surgical patients. Can J Anaesth. 1991; 38:965–968.

17. Goldberg ME, Cantillo J, Larijani GE, Torjman M, Vekeman D, Schieren H. Sevoflurane versus isoflurane for maintenance of anesthesia: are serum inorganic fluoride ion concentrations of concern? Anesth Analg. 1996; 82:1268–1272.
18. Ebert TJ, Arain SR. Renal responses to low-flow desflurane, sevoflurane, and propofol in patients. Anesthesiology. 2000; 93:1401–1406.

20. Ko JS, Gwak MS, Choi SJ, Yang M, Kim MJ, Lee JY, et al. The effects of desflurane and sevoflurane on hepatic and renal functions after right hepatectomy in living donors*. Transpl Int. 2010; 23:736–744.

21. Kim SI, Rha KH, Lee JH, Kim HJ, Kwon KI, Kim YS, et al. Favorable outcomes among recipients of living-donor nephrectomy using video-assisted minilaparotomy. Transplantation. 2004; 77:1725–1728.

22. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999; 130:461–470.

23. Fang ZX, Kandel L, Laster MJ, Ionescu P, Eger EI. Factors affecting production of compound A from the interaction of sevoflurane with Baralyme and soda lime. Anesth Analg. 1996; 82:775–781.

24. Cousins MJ, Mazze RI. Methoxyflurane nephrotoxicity. A study of dose response in man. JAMA. 1973; 225:1611–1616.

25. Mazze RI, Calverley RK, Smith NT. Inorganic fluoride nephrotoxicity: prolonged enflurane and halothane anesthesia in volunteers. Anesthesiology. 1977; 46:265–271.
26. Frink EJ Jr, Malan TP, Atlas M, Dominguez LM, DiNardo JA, Brown BR Jr. Clinical comparison of sevoflurane and isoflurane in healthy patients. Anesth Analg. 1992; 74:241–245.

27. Kharasch ED, Hankins DC, Thummel KE. Human kidney methoxyflurane and sevoflurane metabolism. Intrarenal fluoride production as a possible mechanism of methoxyflurane nephrotoxicity. Anesthesiology. 1995; 82:689–699.
28. Conzen PF, Kharasch ED, Czerner SF, Artru AA, Reichle FM, Michalowski P, et al. Low-flow sevoflurane compared with low-flow isoflurane anesthesia in patients with stable renal insufficiency. Anesthesiology. 2002; 97:578–584.

29. Bito H, Ikeuchi Y, Ikeda K. Effects of low-flow sevoflurane anesthesia on renal function: comparison with high-flow sevoflurane anesthesia and low-flow isoflurane anesthesia. Anesthesiology. 1997; 86:1231–1237.
30. Vats HS, Rayhill SC, Thomas CP. Early postnephrectomy donor renal function: laparoscopic versus open procedure. Transplantation. 2005; 79:609–612.

31. Bito H, Ikeda K. Closed-circuit anesthesia with sevoflurane in humans. Effects on renal and hepatic function and concentrations of breakdown products with soda lime in the circuit. Anesthesiology. 1994; 80:71–76.

32. Moore E, Bellomo R, Nichol A. Biomarkers of acute kidney injury in anesthesia, intensive care and major surgery: from the bench to clinical research to clinical practice. Minerva Anestesiol. 2010; 76:425–440.
33. McIlroy DR, Wagener G, Lee HT. Biomarkers of acute kidney injury: an evolving domain. Anesthesiology. 2010; 112:998–1004.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download