Abstract
Purpose
The aim of this study was to elucidate the effects of immunocompromising comorbidities on treatment response and adverse reactions in older tuberculosis (TB) patients.
Materials and Methods
The medical records of 182 patients older than 65 years with proven TB by positive culture of Mycobacterium tuberculosis and with available drug susceptibility tests were reviewed retrospectively. These patients were subsequently assigned to either the comorbidity group (n=78) or non-comorbidity group (n=104) depending on whether they had immunocompromising comorbidities.
Results
The mean durations of treatment were 9.9±3.3 months in the comorbidity group and 9.3±3.2 months in the non-comorbidity group (p=0.21). M. tuberculosis culture results converted to negative in most patients with available follow-up cultures at two months after treatment. The successful treatment rates were 94.9% and 98.9% in the comorbidity and non-comorbidity groups, respectively (p=0.30). The most common side effects of anti-TB treatment were skin rash/pruritus (13% in the comorbidity group vs. 11% in the non-comorbidity group, p=0.79), gastro-intestinal problems (14% vs. 9%, p=0.25) and hepatotoxicity (14% vs. 7%, p=0.09).
Tuberculosis (TB) is a major cause of illness and death worldwide, especially in developing countries. Even in developed countries where the overall incidence of TB is lower, pulmonary TB remains a public health concern among the elderly.1 Most people (-90%) infected by the tubercle bacillus do not develop disease throughout their lifetime. However, alterations in the immune system, such as in co-infection with HIV or diabetes, increases the risk of developing active disease considerably.2 The cell-mediated immune response is a major immunoprotective mechanism against TB, and patients with comorbidities, which may impair cell-mediated immunity, are at increased risk for developing TB.3 Common immunocompromising conditions include HIV/AIDS, old age, malignancy, immunosuppressive therapy (glucocorticosteroids, chemotherapy, etc.), diabetes mellitus, end-stage renal disease requiring dialysis, malnutrition and liver cirrhosis.4-14 Old age is an important risk factor for TB, and the prevalence of comorbidities that can impair the cell-mediated immune response is much greater in the elderly.15 With a decreasing birth rate and increasing life expectancy, the global population is ageing, and TB in the elderly is becoming clinically important worldwide. However, whether the presence of comorbidities in the elderly affects treatment outcomes from anti-TB therapy has not been evaluated. The aim of this study was to investigate the effects of immunocompromising comorbidities on treatment responses and adverse reactions in TB patients older than 65 years.
A total of 321 patients older than 65 years were diagnosed with TB by positive culture of Mycobacterium tuberculosis from January 2003 to December 2008 at a tertiary care hospital in South Korea. The medical records of 247 of these patients with available drug susceptibility tests were reviewed retrospectively. Only pulmonary TB patients were included, and cases of miliary TB (n=4) or extrapulmonary TB (n=32) were excluded from this study. Twenty-four drug-resistant TB cases, including 12 patients with multi-drug-resistant TB and five patients previously diagnosed with TB at other hospitals, were excluded as well to avoid bias. Finally, 182 older patients who met the above criteria were eligible for inclusion in the analysis. These patients were subsequently assigned into either the comorbidity group (n=78) or non-comorbidity group (n=104) depending on whether they had immunocompromising comorbidities.
The demographic characteristics, presenting symptoms, laboratory results, radiological findings, microbiological study results, adverse reactions to anti-TB treatment and treatment outcomes were recorded from the medical records.
The comorbidities leading to an immunocompromised status in the participants included diabetes mellitus, end-stage renal disease requiring dialysis, chronic hepatitis B or C, liver cirrhosis, malignancy, immunosuppressive therapy (e.g., steroid therapy, chemotherapy), malnutrition, and HIV infection including HIV infection, advanced HIV disease and AIDS. We included patients who were diagnosed with malignancy or who had undergone cancer treatment or immunosuppressive therapy within 6 months of initiation of anti-TB drugs. Malnutrition was defined if more than three of the following criteria were present: serum albumin concentration <3.0 g/dL (reference range: 3.3-5.2 g/dL), absolute lymphocyte count <1.5×103/µL (0.8-4.4×103/µL), cholesterol concentration <3.0 mmol/L (3.0-5.2 mmol/L), and body mass index <18 kg/m2.
Treatment responses were evaluated by examining the culture conversion rate at two months after treatment, treatment duration and treatment outcome. To evaluate treatment duration and outcome, we included only 148 patients (59 patients in the comorbidity group and 89 in the non-comorbidity group) after excluding those who transferred to another institution, did not complete the follow-up, or died from other causes. Treatment outcome was analysed based on the World Health Organization and International Union Against Tuberculosis and Lung Disease recommendations.16 We modified the categories (cured, treatment completed, death, treatment failure, and treatment interrupted) and reclassified them as either "successful treatment" (cured and completed) or "unsuccessful treatment" (death, failure, and interrupted).
The observed treatment-related adverse reactions included hepatotoxicity, skin rash/pruritus, cytopenia, ocular toxicity, general weakness, and gastrointestinal problems including nausea, vomiting and anorexia. Hepatotoxicity was diagnosed when there was an increase in serum alanine aminotransferase or aspartate aminotransferase that was greater than three times the upper limit of the normal or for total bilirubin greater than two times the upper limit of the normal.17
The study was approved by the institutional review board of Seoul National University Hospital (IRB No. 0906-043-283) and was conducted in accordance with the Declaration of Helsinki. Patient consent was not required because this study was performed retrospectively.
Statistical analysis was performed using the software package SPSS® (Version 12.0, Chicago, IL, USA). The χ2 test or Fisher's exact test (if the expected number was <5 in at least one cell) was used to compare categorical variables, and a t test was used to compare continuous variables. Statistical significance was considered if p<0.05.
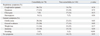
A total of 182 patients who satisfied the inclusion criteria were included in the study; 78 patients were allocated to the comorbidity group and 104 to the non-comorbidity group. The baseline clinical characteristics and laboratory findings of the study population are summarized in Table 1. The median ages of the groups were 71 yr (range: 65-90 yr) and 72 yr (range: 65-87 yr), respectively. The comorbidity group showed a significant male predominance (p<0.01). Thirty-nine patients had a previous history of TB (30% vs. 15%, p=0.02).
In the comorbidity group, 45 patients had diabetes mellitus, 25 patients had malignancies that were detected the first in life time. The detected malignancies were solid tumors, including eight advanced gastric cancers, four lung cancers, two hepatocellular cancers, two colorectal cancers, two hypopharyngeal cancers, and seven other cancers. Additionally, 45% of 25 patients were found to have stage I/II disease, 35% were stage III and 20% were stage IV cancer. Eight patients were treated with surgery, nine were treated with surgery and chemotherapy or radiation, four were treated with radiation therapy, two were treated with chemotherapy and two had no treatment. Seventeen patients experienced malnutrition and 16 patients were receiving immunosuppressive therapy (e.g., corticosteroids, chemotherapy). Two patients had chronic hepatitis B; the durations of HBV infection were 6 and 15 years, respectively. One patient had chronic hepatitis C for more than 10 years. One patient put of three liver cirrhosis cases had chronic hepatitis C. The last two had alcoholic liver cirrhosis. Two patients had end-stage renal disease requiring dialysis. None of the patients had HIV infection. Twenty-eight of 77 patients had more two comorbidities.
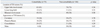
The clinical presentations were similar in both groups except for more frequent febrile sense (36% vs. 21%, p=0.03) and general weakness (30% vs. 16%, p=0.03) in the comorbidity group than in the non-comorbidity group (Table 2). In both groups, the pulmonary TB lesions involved the upper lobe in most patients (Table 3). The percentages of lower lung field TB (15% vs. 11%, p=0.33), bilateral lung involvement (51% vs. 38%, p=0.08), cavitary lesions (24% vs. 27%, p=0.70) and consolidation (37% vs. 30%, p=0.29) did not differ between the two groups.
All patients received anti-TB therapy comprising an isoniazid/rifampicin-based regimen (Table 4). M. tuberculosis culture results were converted to negative in 110 (92%) of 119 patients with available follow-up cultures at two months after treatment [50 (91%) vs. 60 patients (94%), p=0.73]. To evaluate treatment duration and outcomes, we included only 148 patients (59 patients in the comorbidity group and 89 in the non-comorbidity group) by excluding those who transferred to another institution [8 (10%) vs. 9 (9%), p=0.71], did not complete follow-up [5 (6%) vs. 5 (5%), p=0.75] or died from other causes [6 (8%) vs. 1 (1%), p=0.08]. The mean durations of treatment were 9.9±3.3 months in the comorbidity group and 9.3±3.2 months in the non-comorbidity group (p=0.21). The successful treatment rates were 94.9% and 98.9% (p=0.30) in the comorbidity and non-comorbidity groups, respectively (Table 5). Three patients in the comorbidity group failed to achieve bacteriological conversion within 5 months after the start of treatment. TB-related death occurred in only one patient in the non-comorbidity group. Five patients in the comorbidity group and one patient in the non-comorbidity group died due to pneumonia; one patient in the comorbidity died due to underlying malignancy during the follow-up after treatment.
The most common side effects of anti-TB treatment were skin rash/pruritus (13% vs. 11%, p=0.79), gastro-intestinal problems (14% vs. 9%, p=0.25) and hepatotoxicity (14% vs. 7%, p=0.09). The rates of drug-related side effects did not differ between the two groups, although the comorbidity group tended to have more frequent hepatotoxicity (14% vs. 7%, p=0.09) and general weakness (10% vs. 3%, p=0.06) (Table 6). Among the patients with chronic hepatitis B or C or liver cirrhosis, one experienced hepatotoxicity. Thirteen patients (17%) in the comorbidity group and sixteen (16%) in the non-comorbidity group changed anti-TB medication because of drug adverse reactions (p=0.84).
TB is an important cause of morbidity and mortality worldwide, especially in Asia and Africa. The estimates of the global burden of disease caused by TB in 2009 were 9.4 million incident cases (equivalent to 137 cases per 100000 population), 14 million prevalent cases (200 cases per 100000 population) and 1.7 million deaths (26 deaths per 100000 population). Most cases were in Asia (55%) and Africa (30%).18 The Korean National Tuberculosis Association estimates that the overall rate per 100000 in 2010 was 98.4 in the general population, but 266 per 100000 more than 65 years.19 Thus, TB remains a public health problem in the elderly population.
Age-related declines in cell-mediated immunity are known to affect the reactivation of latent infection in the elderly. The prevalence of these immunocompromising comorbidities increases with age, increasing the susceptibility of older patients to TB.15 Many studies on TB in older people have investigated treatment outcomes and adverse effects of pulmonary TB in comparison with these variables in younger populations.5,12-14,20,21 However, only a few studies have compared clinical outcomes and treatment-adverse reactions between TB patients with and without immunocompromising comorbidities.22,23
The present study revealed a high successful treatment rate in these older patients regardless of the existence of immunocompromising comorbidities (94.9% vs. 98.9%). M. tuberculosis culture results were converted to negative in most patients with available follow-up cultures at two months after treatment. The mean duration of treatment did not differ between the comorbidity and non-comorbidity groups (9.9±3.3 months vs. 9.3±3.2 months, p=0.21). The mean treatment duration was much longer than the standard recommended duration of 6 months, possibly because the clinicians may have prescribed a regimen without pyrazinamide for 25-30% of the patients upon concerns for hepatotoxicity in older patients.
Kobashi, et al.22 investigated the clinical features and treatment outcomes of immunocompromised and non-immunocompromised patients with pulmonary TB. The immunocompromised group included patients being treated with immunosuppressive therapies or those having diabetes mellitus, chronic renal failure treated with dialysis, malignant disease, or HIV infection. The responsiveness to medication (good response) did not differ between the immunocompromised patients (86.7%) and non-immunocompromised patients (93.7%), but the TB-related mortality rate was higher in the immunocompromised patients (13.5% vs. 4.5%).22 Kim, et al.7 evaluated the TB treatment responses in patients receiving anticancer chemotherapy and reported a good response rate and no increase in the rate of TB-related deaths in these patients. The treatment responses to anti-TB medication have been reported as fairly good, even in patients with HIV/AIDS.24 By contrast, another study reported unfavourable outcomes in patients with comorbidities.23 The inconsistent results may reflect different definitions of immunocompromising comorbidities, different methods for evaluation of treatment outcomes and different study populations. The study23 that investigated differences between immunocompromised patients and non-immunocompromised patients with tuberculosis defined immunocompromised group as patients that received immunotherapy and chemotherapy, and evaluated treatment outcomes according to sputum negative conversion rate, improvement in symptoms and radiologic findings. On the other hand, our study and Kobashi's study22 included patients with diabetes, ESRD, and malignancy, as well as patients treated with chemotherapy and immunotherapy. In our study, treatment responses were evaluated by examining culture conversion rate, treatment duration and treatment outcome (cure, completion, failure, interruption and TB related death). The consistent risk factors associated with poor TB treatment outcomes were being male and of older age.25-27 Some reports also suggest that comorbidities are risk factors.12,27,28 However, we could not confirm differences in treatment outcomes between patients with and without comorbidities in patients older than 65 years.
A few limitations of the study warrant consideration. This study was underpowered due to the small sample size, and the small sample size may have failed to detect any differences between two groups. The retrospective study design also has limitations in the possibility of non-random missing in the analysis of sputum negative conversion and treatment outcomes.
Kobashi, et al.22 reported no significant difference in the rates of drug-related side effects between groups, a finding that is consistent with our data. However, the comorbidity group tended to have more frequent hepatotoxicity (14% vs. 7%, p=0.09) and general weakness (10% vs. 3%, p=0.06). Concomitant treatment with anti-TB drugs, other medications for coexisting diseases, and malnutrition in the comorbidity group may have contributed to the higher prevalence of hepatotoxicity. Hepatotoxic reactions to commonly used anti-TB drugs, such as isoniazid, rifampicin, and pyrazinamide, often pose a problem in the clinical management of older TB patients.12 As mentioned above, the risk of hepatic dysfunction in older people may explain the lower rate of use of pyrazinamide for treating TB in the older patients in this study.
In brief, we found that treatment responses, reflected by a negative conversion rate of culture, duration of treatment and treatment outcome (successful treatment rate vs. unsuccessful treatment rate), as well as treatment-related adverse effects, were not affected by the presence of immunocompromising comorbidities in older patients.
The present study shows that the successful treatment rate is high and that immunocompromising comorbidities have no effect on responses to treatment and incidence of adverse effects in older TB patients.
Figures and Tables
Table 1
Baseline Demographic and Clinical Characteristics of the Patients with Pulmonary Tuberculosis

References
1. Rajagopalan S. Tuberculosis and aging: a global health problem. Clin Infect Dis. 2001; 33:1034–1039.

2. Hernandez-Pando R, Orozco H, Aguilar D. Factors that deregulate the protective immune response in tuberculosis. Arch Immunol Ther Exp (Warsz). 2009; 57:355–367.

3. North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004; 22:599–623.
4. FitzGerald JM, Grzybowski S, Allen EA. The impact of human immunodeficiency virus infection on tuberculosis and its control. Chest. 1991; 100:191–200.

5. Pérez-Guzmán C, Vargas MH, Torres-Cruz A, Villarreal-Velarde H. Does aging modify pulmonary tuberculosis?: a meta-analytical review. Chest. 1999; 116:961–967.
6. Ibrahim EM, Uwaydah A, al-Mulhim FA, Ibrahim AM, el-Hassan AY. Tuberculosis in patients with malignant disease. Indian J Cancer. 1989; 26:53–57.
7. Kim DK, Lee SW, Yoo CG, Kim YW, Han SK, Shim YS, et al. Clinical characteristics and treatment responses of tuberculosis in patients with malignancy receiving anticancer chemotherapy. Chest. 2005; 128:2218–2222.

8. Singla R, Khan N, Al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis. 2006; 10:74–79.
9. Choudhury D, Luna-Salazar C. Preventive health care in chronic kidney disease and end-stage renal disease. Nat Clin Pract Nephrol. 2008; 4:194–206.

11. Thulstrup AM, Mølle I, Svendsen N, Sørensen HT. Incidence and prognosis of tuberculosis in patients with cirrhosis of the liver. A Danish nationwide population based study. Epidemiol Infect. 2000; 124:221–225.

12. Leung CC, Yew WW, Chan CK, Chau CH, Tam CM, Lam CW, et al. Tuberculosis in older people: a retrospective and comparative study from Hong Kong. J Am Geriatr Soc. 2002; 50:1219–1226.

13. Rocha M, Pereira S, Barros H, Seabra J. Does pulmonary tuberculosis change with ageing? Int J Tuberc Lung Dis. 1997; 1:147–151.
14. Teale C, Goldman JM, Pearson SB. The association of age with the presentation and outcome of tuberculosis: a five-year survey. Age Ageing. 1993; 22:289–293.

15. Vesosky B, Turner J. The influence of age on immunity to infection with Mycobacterium tuberculosis. Immunol Rev. 2005; 205:229–243.

16. Veen J, Raviglione M, Rieder HL, Migliori GB, Graf P, Grzemska M, et al. Standardized tuberculosis treatment outcome monitoring in Europe. Recommendations of a Working Group of the World Health Organization (WHO) and the European Region of the International Union Against Tuberculosis and Lung Disease (IUATLD) for uniform reporting by cohort analysis of treatment outcome in tuberculosis patients. Eur Respir J. 1998; 12:505–510.
17. Shang P, Xia Y, Liu F, Wang X, Yuan Y, Hu D, et al. Incidence, clinical features and impact on anti-tuberculosis treatment of anti-tuberculosis drug induced liver injury (ATLI) in China. PLoS One. 2011; 6:e21836.

18. World Health Organization. Global tuberculosis control. WHO Report 2010. Geneva, Switzerland: World Health Organization;2010.
19. Korea Centers for Disease Control and Prevention. Annual report on the notified tuberculosis cases patients in Korea 2010. Seoul: Korea Centers for Disease Control and Prevention;2011.
20. Lee JH, Han DH, Song JW, Chung HS. Diagnostic and therapeutic problems of pulmonary tuberculosis in elderly patients. J Korean Med Sci. 2005; 20:784–789.

21. Korzeniewska-Kosela M, Krysl J, Müller N, Black W, Allen E, FitzGerald JM. Tuberculosis in young adults and the elderly. A prospective comparison study. Chest. 1994; 106:28–32.
22. Kobashi Y, Mouri K, Yagi S, Obase Y, Miyashita N, Okimoto N, et al. Clinical features of immunocompromised and nonimmunocompromised patients with pulmonary tuberculosis. J Infect Chemother. 2007; 13:405–410.

23. Shao C, Qu J, He L. A comparative study of clinical manifestations caused by tuberculosis in immunocompromised and non-immunocompromised patients. Chin Med J (Engl). 2003; 116:1717–1722.
24. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003; 167:603–662.

25. Cegolon L, Maguire H, Mastrangelo G, Carless J, Kruijshaar ME, Verlander NQ. Predictors of failure to complete tuberculosis treatment in London, 2003-2006. Int J Tuberc Lung Dis. 2010; 14:1411–1417.
26. Baussano I, Pivetta E, Vizzini L, Abbona F, Bugiani M. Predicting tuberculosis treatment outcome in a low-incidence area. Int J Tuberc Lung Dis. 2008; 12:1441–1448.
27. Muñoz-Sellart M, Cuevas LE, Tumato M, Merid Y, Yassin MA. Factors associated with poor tuberculosis treatment outcome in the Southern Region of Ethiopia. Int J Tuberc Lung Dis. 2010; 14:973–979.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download