Abstract
Purpose
Lactobacillus casei (L. casei) is known to exert anti-proliferation effects on many types of cancer cells. However, the effect of L. casei on liver cancer has not been reported. Accordingly, the aim of this study was to determine the anti-cancer effect of L. casei extract on Huh7 cells.
Materials and Methods
L. casei ATCC393 extract was prepared and purified. After the treatment of L. casei extract on Huh7 cells, cell viability, cell cycle arrest and cell death were analyzed by flow cytometry. The expression levels of tumor necrosis factor-α receptor 1 (TNFR1) and death receptor 3 (DR3) mRNA related with extrinsic apoptosis were assessed by reverse transcription polymerase chain reaction. Additionally, P21 and P27 cell cycle proteins as well as Caspase-3, -8, -9, phospho-Bad and Bcl-2 apoptosis proteins were analyzed by western blot analysis. To determine the effect of L. casei extract on cancer stem-like cells, we analyzed changes in side population fraction through flow cytometry.
Results
The cell viability of Huh7 cells treated with L. casei extract was decreased by 77%, potentially owing to increases in the rates of Huh7 cells arrested in the G2/M phase (3% increase) and that underwent apoptosis (6% increase). The expression levels of TNFR1 and DR3 mRNA, as well as P21 and P27 cell cycle proteins, were increased. Meanwhile, the expressions of caspase-8, -9, phospho-Bad and Bcl-2 proteins decreased. However, in the case of side population cells, no remarkable changes were observed.
Lactobacillus casei (L. casei) is a gram-positive non-pathogenic, rod-shaped anaerobic lactic acid bacterium, which is widely distributed in nature and commonly present in the human mouth, intestines and reproductive tracts. L. casei is now used widely in nutritional supplements and beneficially affects the host by improving its intestinal microbial balance in the human body. Previously, L. casei was shown to induce improvement in murine models of inflammatory disorders such as arthritis, type I diabetes and systemic lupus, as well as murine chronic colitis and ileitis.1,2 Oral administration of L. casei exhibits anti-tumor activity by augmentation of the host's immune system because of the strain's immunomodulatory activity.3 Furthermore, a cell wall-derived polysaccharide-peptidoglycan complex in one L. casei strain plays a role in its anti-inflammatory actions in chronic intestinal inflammatory disorders.4 Moreover, L. casei has the ability to moderate inflammatory responses in damaged liver tissue.5 In a previous study, children treated with L. casei after liver transplantation experienced beneficial effects limited to the period of bacteria intake.6
Several Lactobacillus strains have been shown to exert potent anti-tumor effects in rodents.7 L. casei was reported to potentiate systemic immune responses in colon cancer mouse models,8 and also to reduce tumor induction upon oral administration.9 In another study, L. casei induced an immune response and tumoricidal growth inhibition upon intraperitoneal (i.p.) inoculation.10 Orally administered L. casei is also known to be useful in preventing the recurrence of superficial bladder cancer.11,12 Previous studies demonstrated that L. casei may retain its anti-cancer effects in human cancers. However, the anti-tumor activity of L. casei against liver cancer has not yet been reported.
Hepatocellular carcinoma is one of the most common cancers in the world and its prognosis is very poor. Nevertheless, administration of food supplements may be an easy and effective way of decreasing the incidence of liver cancer. L. casei is widely used in supplemental health foods, and has been shown to alleviate liver injury in rat models and maintain the normal structure of hepatocytes.13
In this study, we investigated the anti-tumor activity of L. casei against human liver cancer cells. In doing so, we validated the efficacy of L. casei in Huh7 human hepatocellular carcinoma cells directly treated with L. casei extract. Through this study, L. casei extract was found to inhibit the growth of Huh7 cells.
L. casei ATCC393 (American Type Culture Collection) was obtained from the Korean Culture Center of Microorganisms. The bacteria were grown in Lactobacillus Man-Rogosa-Sharp (MRS) broth (Difco0881) at 37℃. The growth media consisted of 10 g of peptone, 10 g of beef extract, 5 g of yeast extract, 20 g of glucose, 1 g of sorbitan monooleate complex (Tween 80), 5 g of ammonium acetate, 5 g of sodium acetate, 2 g of K2HPO4, 0.2 g of MgSO4-7H2O, and 0.2 g of MnSO4-4H2O. The pH of the growth medium was adjusted to 6.5 after mixing the components.
L. casei was grown in 1 liter MRS medium for 24 hrs up to the mid-exponential phase, an A.600 value of about 0.5. After three washings with 1X phosphate buffered saline (PBS), bacterial cell walls were disrupted with a Sonic dismembrator (Fisher scientific, Rockingham County, NH, USA) for 1 minute on ice. The extracted protein concentration was assayed by the Quick Start Bovine Serum Albumin (BSA) Standard kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's guidelines.
Huh7, human hepatoma cells were cultured in Dulbecco's modified Eagle's medium (WelGene, Daegu, Korea) containing 10% fetal bovine serum (GibcoBRL, Grand Island, NY, USA). Cells were seeded on cell culture dishes (Falcon, San Jose, CA, USA) and sub-cultured by trypsin-EDTA (GibcoBRL, Grand Island, NY, USA) treatment. All cells were maintained at 37℃ in a humidified 5% CO2 atmosphere.
Huh7 cells were seeded into cell culture plates at a density of 5×105 cells. The effect of L. casei extract on Huh7 cells viability was compared and determined by inverted microscopy (Olympus Model IX51, Tokyo, Japan), comprising a DP50 camera system (Olympus, Tokyo, Japan), using a 0.4% trypan blue dye exclusion method 72 hours after treatment with the 0.1 mg concentration. Cell survival was evaluated on the basis of the percentage of the total number of Huh7 cells (viable plus nonviable). Mean survival rate was determined by counting randomly selected non-overlapping fields four times. Each culture dish represents an n=1 determination with each experiment replicated independently 4 to 6 times using different cultures.
Huh7 cells were treated with 0.1 mg L. casei extract for 72 hours, harvested, fixed with 70% ethanol for 15 minutes, thawed and centrifuged at 1200 rpm for five minutes, washed with 1 mL of ice-cold 1X PBS, centrifuged again, and then treated with 100 µg/mL of RNAase A (Sigma, St. Louis, MO, USA) for 90 minutes at 37℃ in a humidified 5% CO2 atmosphere. Cells were stained with 25 µg/mL propidium iodide (Sigma, St. Louis, MO, USA) to analyze cell cycles. Cells were analyzed using a FACSCalibur (Becton Dickinson, San Jose, CA, USA), which indicated the distribution of the cells into their respective cell-cycle phases based on their DNA content. G0/G1 cells were 2n, S-phase cells were >2n, but <4n, and G2/M cells were 4n. All experiments were performed in triplicate.
Huh7 cells were cultured as described above. Annexin V staining was done using an Annexin V-FITC Apoptosis kit (BD Pharmingen, Franklin Lakes, NJ, USA), according to the manufacturer's protocol. Annexin V-positive cells were measured at a fluorescence intensity of 1×104 cells using the FACSCalibur system (Becton & Dickinson, San Jose, CA, USA) within 1 hour.
Cytoplasmic RNAs were extracted from Huh7 cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) to evaluate expression changes in tumor necrosis factor-α receptor 1 (TNFR1), death receptor 3 (DR3) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA after treatment of the cells with 0.1 mg of L. casei extract for 72 hrs. The first-strand cDNA was generated using EcoDry Premix RT kits (oligo dT, random hexamer double primed, Clontech, CA, USA) and synthesized from 1 µg of total RNA according to the manufacturer's instructions. The primer sequences used were as follows: TNFR1 (forward: 5'-GAA ATG GGT CAG GTG GAG ATC T-3', reverse: 5'-GTT CTT CCT GCA GCC ACA ATC T-3'),14 DR3 (forward: 5'-TCT TCA CCC CCT CTC GAC AT-3', reverse: 5'-TTC CGA GAC CAG CAG TAC GA-3'),15 and GAPDH (forward: 5'-GAGCCACATCGCTCAGAC-3', reverse: 5'-CTTCTCATGGTTCACACCC-3').16 PCR reaction mixtures contained 10 pmol of each primer, and comprised a total reaction volume of 20 µL. Amplification was performed for 40 cycles for TNFR1, 36 cycles for DR3, and 21 cycles for GAPDH using an T3000 Thermal cycler instrument (Biometra, Goettingen, Germany). PCR products were analyzed by 1.5% agarose gel electrophoresis and then visualized by a UV trans-illuminator (Gel-Doc, Bio-rad, Hercules, CA, USA).
The following primary antibodies were used for Western blot analysis: antibodies against P21 (1 : 1000; Cell Signaling Technology, Danvers, MA, USA), P27 (1 : 1000; Cell Signaling Technology, Danvers, MA, USA), caspase-3 (1 : 2000; Calbiochem, Darmstadt, Germany), caspase-8 (1 : 1000; Calbiochem, Darmstadt, Germany), caspase-9 (1 : 2000; Calbiochem, Darmstadt, Germany), phospho-Bad (1 : 2000; Cell Signaling Technology, Danvers, MA, USA), Bcl-2 (1 : 1000; Cell Signaling Technology, Danvers, MA, USA), and human actin (1 : 1000; Cell Signaling Technology, Danvers, MA, USA). Huh7 cells were lysed with 1X RIPA lysis buffer (Cell Signaling Technology, Danvers, MA, USA). The total protein concentration was determined with a Quick Start Bovine Serum Albumin Standard kit (Bio-rad, Hercules, CA, USA). Equal amounts of proteins were loaded onto a sodium dodecyl sulfate poly-acrylamide 4-12% gradient gel (Invitrogen, Carlsbad, CA, USA), electrophoretically separated for 2 hours at 180V with a X-cell kit (Invitrogen, Carlsbad, CA, USA), transferred onto a polyvinylidene difluoride (PVDF) membrane (Invitrogen, Carlsbad, CA, USA), incubated with 5% w/v nonfat dry milk in tris-buffered saline tween 20 (TBST) buffer for 1 hour at room temperature, and probed with primary antibodies overnight at 4℃. After incubation with horseradish peroxidase conjugated goat anti-mouse and anti-rabbit secondary antibodies (1 : 2000) (Cell Signaling Technology, Danvers, MA, USA), peroxidase activity was detected by a LumiGLO chemiluminescent substrate system (KPL, Gaithersburg, MD, USA), according to the manufacturer's instructions.17 Human actin levels were used as a control.
Side population cells were analyzed as previously described.18 To determine the effect of L. casei extract on side population cells, Huh7 cells were cultured and harvested as described previously. To analyze side populations, cells were incubated with Hoechst 33342 5 µg/mL (Sigma, St. Louis, MO, USA) or Hoechst 33342 5 µg/mL and verapamil 50 µmol/L (Sigma, St. Louis, MO, USA) for 90 minutes at 37℃. Cells were vortexed every 15 minutes. Propidium iodide 1 µg/mL (Sigma, St. Louis, MO, USA) was added to identify dead cells at the end of the incubation period. Side population cells were detected by FACS Aria (Becton & Dickinson, San Jose, CA, USA), using a 515-nm side population filter (Hoechst blue).
All experiments were performed independently in triplicate. All data were calculated using the R-statistics program. Data were presented as mean±standard error, and Student's t-test was used to evaluate differences between the treated and control experiments. p-values <0.05 were considered statistically significant.
To investigate the effect of L. casei extract on liver cancer cell lines, Huh7 cells were treated with an optimal concentration of L. casei extract of 0.1 mg, and the growth rate was evaluated using a 0.4% trypan blue dye exclusion method. Huh7 cells treated with L. casei extract were not significantly different compared with the controls after 24 hours. As shown in Fig. 1, the growth rate of L. casei extract-treated Huh7 cells was considerably lower (77%), compared to the controls. Thus, L. casei extract was specifically effective against liver cancer cells.
To study the effect of L. casei extract on the different phases of the cell cycle, the DNA contents of Huh7 cells treated with L. casei extract were analyzed by flow cytometry after staining with propidium iodide and compared to that of the controls. As shown in Fig. 2, L. casei extract-treated Huh7 cells displayed a distribution of 42% in G0/G1, 41% in the S phase, and 17% in G2/M. The cell-cycle distribution of Huh7 control group cells was 43% in G0/G1, 43% in the S phase, and 14% in G2/M. L. casei extract increased the rate of arrested Huh7 cells in the G2/M phase by 3% compared to the control group.
To determine whether the effect of L. casei extract on growth inhibition was due to cell death, as well as cell cycle arrest, the extent of cell death was determined by staining with Annexin-V-FITC and propidium iodide together, 72 hours after the treatment of Huh7 cells with L. casei extract. After treating cells with L. casei extract, 2% of cells were found in early apoptosis (lower, right) and 4% of cells were in late apoptosis (upper right and left). The cell death rate was found to be increased by 6% in the treated group, compared to the control group (Fig. 3).
Tumor necrosis factor-α receptor 1 [TNFR1; also known as death receptor 1 (DR1)] and DR3 (also known as APO-3, TRAMP) play roles of inducing apoptosis. These TNF receptors induce apoptosis by extracellular signals. Compared to the control group, L. casei extract-treated Huh7 cells exhibited significant increases in mRNA expression levels of TNFR1 and DR3 (Fig. 4). Thus, L. casei extract increased apoptosis in Huh7 cells through multiple extracellular related death receptors.
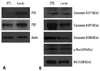
L. casei extract increased the expression levels of P21 and P27 proteins in Huh7 cells (Fig. 5A). P21 is a cyclin-dependent kinase inhibitor and inhibits the activity of cyclin-dependent kinase (CDK)-1, -2 complex. The function of P21 is responsible for inhibiting cell growth and inducing cells to apoptosis. Expression of P21 is dependent on extrinsic stimulus and thus prevents cell proliferation. P27 also acts as a tumor suppressor protein that inhibits CDK complexes and plays a role as a cell cycle regulator. L. casei extract was found to decrease the viability of Huh7 cells by inducing P21 and P27-dependent cell cycle arrest.
Caspase-3, caspase-8 and caspase-9 play important roles in mitochondria-related intrinsic and death receptor-related extrinsic pathways of apoptosis. Caspase-3 is the final executioner of apoptosis, while caspase-8 and -9 are mediators of the intrinsic mitochondrial pathway of apoptosis. As shown in Fig. 5B, caspase-3 levels were unchanged, while both caspase-8 and -9 levels decreased after treatment of Huh7 cells with L. casei extract. Additionally, phospho-Bad, an anti-apoptotic protein associated with the cleavage of pro-caspase-9, and Bcl-2, an apoptosis regulator protein, were also decreased. Thus, Huh7 cells treated with L. casei extract were induced apoptosis by caspase-8, -9, phospho-Bad, Bcl-2 decreasing manner.
Side population (SP) cells have been identified as cancer stem-like cells (CSC) in leukemia and solid cancer cells. SP cells demonstrate specific characteristics of self-renewal, differentiating, tumor initiating and drug resistance. To determine the effect of L. casei extract on the viability of side population cells, we analyzed changes in side population fractions through Hoechst 33342 staining by flow cytometry before and after treatment of L. casei cellular extract in Huh7 cells. Accordingly, L. casei extract was found to increase side population fractions by 0.5% in the treated group (2.3%), compared to the control (1.8%). Thus, L. casei extract failed to inhibit CSC in Huh7 cells.
Lactic acid bacteria, such as Lactobacillus strains, have been reported to possess certain anticancer properties.19 However, the anti-tumor activity of L. casei again human liver cancer has not been reported. So, in the present study, we evaluated the inhibitory effects of L. casei on Huh7 human hepatoma cells to demonstrate the causes of such effects by the trypan blue dye exclusion method and flow cytometry after staining with Annexin-V-FITC and propidium iodide together. We found that L. casei extract decreased the cell viability of Huh7 cells by 77%, compared to the controls, due to both cell cycle arrest in the G2/M phase and apoptosis.
In the present, we used the whole cellular extract of L. casei to evaluate anti-tumor activity, without differentiating cytoplasm components and cell wall components. Peptidoglycans and membrane components derived from the cell wall of L. casei were reported to have anti-cancer activities.20,21 Additionally, the soluble polysaccharide fraction of cellular extracts of Lactobacillus strains was found to inhibit cancer cell proliferation.19 Taken together, we concluded that the cell wall components of L. casei exhibit anti-tumor activities. Both chemotherapy and radiotherapy provide beneficial effects and improve quality of life and survival for two thirds of cancer patients. However, these therapies are temporal in many cases because of remission or subsequent relapses of cancer.21 Recently, evidence has suggested that tumors are composed of a heterogeneous cell population with a small subset of CSC that sustain tumor formation and growth. The remission of drug resistance has recently been explained, by the discovery that CSC have resistance against anti-cancer drugs.22 CSC are found to be enriched in a side population of cells that can recognized by fluorescence-activated cell sorting (FACS).18 To date, side population cells have been detected in various solid tumor cells.23 In the present study, after the treatment of L. casei extract, we used dual-wavelength flow cytometry to detect Huh7 side population cells on the basis of the ability of these cells to efflux the fluorescence DNA-binding dye Hoechst 33342. We found in this study that the fraction of side population cells in the treated group was higher than that in the control group (Fig. 6). This means that L. casei extract did not affect cell viability in side population cells. Side population cells are known to have high drug efflux capacity with strong expression of the ATP-binding cassette transporter protein ABCG2/Bcrp1.24 Accordingly, the bacterial extracts might not have affected the Huh7 side population cells because of the active efflux pump.
Previous studies reported that both P21 and P27 are cell cycle arrest related proteins.25 P21 is known to inhibit cell growth and be related to apoptosis. P27 acts as a cell cycle regulator. The elevation of these proteins indicates the induction of cell cycle arrest. In Huh7 cells treated with L. casei extract, the expression levels of P21 and P27 proteins were increased, indicating that the treatment of L. casei extract is related to P21 and P27 dependent cell cycle arrest.
We also found that the expression of DR3 mRNA is increased by L. casei treatment in Huh7 cells. This evidence demonstrated that L. casei induced apoptosis is mediated through DR3 dependent pathways. TNFR1,26 DR315 is a protein expressed in various cell lines and mediates the effect of TNF-a. TNFR1 mRNA was also increased in Huh7 cells treated with L. casei extract, indicating that TNFR1 is also related to extrinsic apoptosis pathways. Thus, L. casei extract increases the apoptosis of Huh7 cells through multiple extracellular mechanisms.
Caspase-3, -8 and -9 play crucial roles in the apoptotic pathway. In the present study, caspase-3 was not significantly decreased after treatment of L. casei extract in Huh7 cells, while caspase-8 and -9, as well as phospho-Bad and Bcl-2 were. Thus, Huh7 cells treated with L. casei extract were induced by apoptosis pathway by caspase-8, -9, phospho-Bad, Bcl-2 decreasing mechanisms.
Finally, we demonstrated that L. casei extract is a promising candidate for treating hepatocellular carcinoma, and some of the small molecules of L. casei may be of use in anti-cancer drug components.
Figures and Tables
Fig. 1
Cell growth rate following the treatment of Lactobacillus casei extract. Huh7 cells seeded at 5×105 cells per cell culture dish and treated with 0.1 mg L. casei extract for 72 hrs. The values are presented in means and standard errors for at least three independent experiments. *p value <0.01. CTL, control.
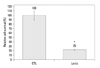
Fig. 2
Cell cycle arrest following the treatment of Lactobacillus casei extract. L. casei extract increased the rate of arrested Huh7 cells in the G2/M phase by 3%, compared to the control group. The data shown are one representative experiment from a total of three independent experiments. CTL, control.

Fig. 3
Flow cytometry of PI-Annexin-V. The cell death rate was found to increased by 6% in the 0.1 mg L. casei extract treated group, compared to the control group, after 72 hrs. The data shown are one representative experiment from a total of three independent experiments. Statistical differences between numbers of apoptotic cells were observed with p<0.05. FL1, fluorescence channel 1; FL3, fluorescence channel 3; FITC, fluorescein isothiocyanate; PI, propidium iodide.

Fig. 4
Expression level of TNFR1 and DR3 mRNA. Significant increments in the mRNA expression levels of TNFR1 and DR3 were observed in L. casei extract treated Huh7 cells. GAPDH mRNA expression was used as a control. TNFR1, tumor necrosis factor-α receptor 1; DR3, death receptor 3; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. CTL, control.
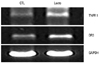
Fig. 5
Western blot analysis of cell cycle and apoptosis related proteins. (A) Expression levels of P21 and P27 cell cycle proteins were increased in Huh7 cells treated with L. casei extract. The increments in P21 and P27 protein expression levels were related with the induction of cell cycle arrest. (B) Expression levels of apoptosis proteins, including caspase-3, caspase-8, caspase-9, phopho-Bad and Bcl-2, were decreased when treated with L. casei extract. Actin protein was used as an internal control. CTL, control.
ACKNOWLEDGEMENTS
This work was supported by the Graduate School of Specialization for Biotechnology Program of the Ministry of Knowledge Economy (MKE) (NO.C-7010-1102-0001).
References
1. Kato I, Endo-Tanaka K, Yokokura T. Suppressive effects of the oral administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci. 1998; 63:635–644.

2. Matsuzaki T, Nagata Y, Kado S, Uchida K, Kato I, Hashimoto S, et al. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei. APMIS. 1997; 105:643–649.

3. Matsuzaki T, Takagi A, Ikemura H, Matsuguchi T, Yokokura T. Intestinal microflora: probiotics and autoimmunity. J Nutr. 2007; 137:3 Suppl 2. 798S–802S.

4. Matsumoto S, Hara T, Nagaoka M, Mike A, Mitsuyama K, Sako T, et al. A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology. 2009; 128:1 Suppl. e170–e180.
5. Haro C, Zelaya H, Lazarte S, Alvarez S, Agüero G. Lactobacillus casei: influence on the innate immune response and haemostatic alterations in a liver-injury model. Can J Microbiol. 2009; 55:648–656.

6. Pawłowska J, Klewicka E, Czubkowski P, Motyl I, Jankowska I, Libudzisz Z, et al. Effect of Lactobacillus casei DN-114001 application on the activity of fecal enzymes in children after liver transplantation. Transplant Proc. 2007; 39:3219–3221.

7. Kato I, Kobayashi S, Yokokura T, Mutai M. Antitumor activity of Lactobacillus casei in mice. Gann. 1981; 72:517–523.
8. Kato I, Endo K, Yokokura T. Effects of oral administration of Lactobacillus casei on antitumor responses induced by tumor resection in mice. Int J Immunopharmacol. 1994; 16:29–36.

9. Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Inhibitory effect of oral administration of Lactobacillus casei on 3-methylcholanthrene-induced carcinogenesis in mice. Med Microbiol Immunol. 1999; 188:111–116.

10. Kato I, Yokokura T, Mutai M. Induction of tumoricidal peritoneal exudate cells by administration of Lactobacillus casei. Int J Immunopharmacol. 1985; 7:103–109.

11. Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. The BLP Study Group. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. Eur Urol. 1995; 27:104–109.

12. Nanno M, Kato I, Kobayashi T, Shida K. Biological effects of probiotics: what impact does Lactobacillus casei shirota have on us? Int J Immunopathol Pharmacol. 2011; 24:1 Suppl. 45S–50S.
13. Hathout AS, Mohamed SR, El-Nekeety AA, Hassan NS, Aly SE, Abdel-Wahhab MA. Ability of Lactobacillus casei and Lactobacillus reuteri to protect against oxidative stress in rats fed aflatoxins-contaminated diet. Toxicon. 2011; 58:179–186.

14. Wang DH, Koehler SM, Mariash CN. Detecting graves' disease: presentations in young athletes. Phys Sportsmed. 1996; 24:35–40.
15. Zhang L, Zhang Y, Zhang L, Yang X, Lv Z. Lupeol, a dietary triterpene, inhibited growth, and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Invest. 2009; 27:163–170.

16. Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY, et al. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. J Natl Cancer Inst. 2008; 100:359–372.

17. Chilampalli C, Guillermo R, Kaushik RS, Young A, Chandrasekher G, Fahmy H, et al. Honokiol, a chemopreventive agent against skin cancer, induces cell cycle arrest and apoptosis in human epidermoid A431 cells. Exp Biol Med (Maywood). 2011; 236:1351–1359.

18. Kim JB, Ko E, Han W, Shin I, Park SY, Noh DY. Berberine diminishes the side population and ABCG2 transporter expression in MCF-7 breast cancer cells. Planta Med. 2008; 74:1693–1700.

19. Choi SS, Kim Y, Han KS, You S, Oh S, Kim SH. Effects of Lactobacillus strains on cancer cell proliferation and oxidative stress in vitro. Lett Appl Microbiol. 2006; 42:452–458.

20. Fichera GA, Giese G. Non-immunologically-mediated cytotoxicity of Lactobacillus casei and its derivative peptidoglycan against tumor cell lines. Cancer Lett. 1994; 85:93–103.

21. Kim JY, Woo HJ, Kim YS, Kim KH, Lee HJ. Cell cycle dysregulation induced by cytoplasm of Lactococcus lactis ssp lactis in SNUC2A, a colon cancer cell line. Nutr Cancer. 2003; 46:197–201.

22. Hosonuma S, Kobayashi Y, Kojo S, Wada H, Seino K, Kiguchi K, et al. Clinical significance of side population in ovarian cancer cells. Hum Cell. 2011; 24:9–12.

23. Moserle L, Ghisi M, Amadori A, Indraccolo S. Side population and cancer stem cells: therapeutic implications. Cancer Lett. 2010; 288:1–9.

24. Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct "side population" of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004; 101:14228–14233.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download