Abstract
Purpose
Sorafenib is an effective systemic agent for advanced hepatocellular carcinoma. To increase its efficacy, we evaluated the feasibility and benefit of sorafenib combined with radiotherapy.
Materials and Methods
From July 2007 to July 2011, 31 patients were treated with a daily dose of 800 mg of sorafenib and radiotherapy. Among them, 13 patients who received radiotherapy on the bone metastasis were excluded. Thirteen patients received 30-54 Gy of radiotherapy on the primary tumor (primary group) and 5 patients received 30-58.4 Gy on the measurable metastatic lesions (measurable metastasis group). Tumor responses at 1 month after the completion of radiotherapy and overall survival were evaluated.
Results
The in-field response rate was 100% in the primary group and 60% in the measurable metastasis group. A decrease of more than 80% in the tumor marker α-fetoprotein was observed in 7 patients in the primary group (54%). Toxicities of grades 3-4 were hand-foot syndrome in 3 (17%) patients, duodenal bleeding in 1 (6%) patient, thrombocytopenia in 3 (17%) patients and elevation of aspartate transaminase in 1 (6%) patient. The median overall survival was 7.8 months (95% confidence interval, 3.0-12.6).
Conclusion
The combined treatment of sorafenib and radiotherapy was feasible and induced substantial tumor responses in the target lesions. The results of this study emphasize the importance of individualized approach in the management of advanced hepatocellular carcinoma and encourage the initiation of a controlled clinical trial.
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide.1 The incidence is also increasing, not only in Asia by hepatitis B viral infection, but also in the United States, Europe, and Japan by hepatitis C infection.2,3 In addition, liver cirrhosis due to alcoholic liver disease and/or genetic problems also poses a risk of HCC.4,5 However, approximately 70% of patients are diagnosed with advanced stage diseases, and treatment options for them are very limited.6
Sorafenib (Nexavar, Bayer HealthCare Pharmaceutics, Leverkusen, Germany-Onyx Pharmaceuticals, South San Francisco California, CA, USA) is an oral multi-kinase inhibitor that blocks the serine-threonine kinase Raf-1 and the activity of receptor tyrosine kinases of vascular endothelial growth factor receptors,7,8 which are key mediators in the molecular pathogenesis of HCC.9-12 It has shown survival benefit in advanced HCC patients through randomized phase III trials.13,14 A meta-analysis of randomized controlled trials confirmed significant prolongation of time to progression by 79% and an increase in overall survival by 37.3%.15 Based on this evidence, sorafenib has been the first and the only systemic agent for advanced HCC. However, there are not many studies that have investigated the use of combined treatment modalities to enhance the efficacy of sorafenib.
Radiotherapy (RT) is one of possible candidates. The National Comprehensive Cancer Network Guidelines version 2.2012 of HCC recommends RT as a locoregional therapy for all tumors irrespective of location.16 In addition, there are several studies revealing good responses and feasibilities of combining RT with other treatment modalities.17,18
The antiangiogenic effects of sorafenib may contribute to escalating the radiosensitivity of the tumor by improving tumor oxygenation. In addition, the antiproliferative effects of sorafenib may delay disease progression outside the radiation field. On the other hand, RT to the target lesion can enhance overall response rates (ORR) in patients treated with sorafenib by reducing local tumor burden.
Based on these rationales, a combined treatment of sorafenib and RT may be a novel therapeutic strategy with stronger antitumor effects. Until now, the clinical experience using this combined treatment has been limited. In this study, we aimed to evaluate feasibility of combined treatment of sorafenib and RT in locally advanced or metastatic HCC. Also, we examined the response and survival time to estimate the efficacy of the combined treatment.
This study was approved by the Institutional Review Board of our institution (approval number: 4-2010-0761) and revised for extension of the period to 2012.
Between July 2007 and July 2011, 31 HCC patients were treated concurrently with sorafenib and RT. The medical records, results of laboratory tests, histology, and imaging studies of these patients were retrospectively reviewed. After the review, 13 patients who received RT on the bone metastasis were excluded, and remaining 18 patients were analyzed.
Diagnosis of HCC was based on either histologic proof (6 patients) or clinicoradiological criteria under the practice guidelines of the Korean Liver Cancer Study Group (12 patients).19
Patients were divided into two groups according to the site of RT: the primary group for primary liver lesions (13 patients), and the measurable metastasis group for mass forming measurable metastatic lesions (5 patients). Treatment decisions were made through discussion at multidisciplinary conference of liver cancer special clinic. Every patient received full explanation of the discussion. General inclusion criteria of primary group were 1) locally advanced stage; 2) unable to perform intra-arterial chemotherapy because of tumor thrombosis or vessel invasion; and 3) patients with metastasis but the tumor burden could be covered by radiation or main tumor burden was inside or near the liver. General inclusion criteria of measurable metastasis group were 1) recurred with measurable metastasis which could be covered by RT; and 2) liver was cancer-free.
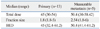
Table 1 shows the clinical features of the patients. The median age of the patients was 53 years. Each patient's stage was determined using the Japanese Tumor-Node-Metastasis system.20 Eighty-three percent of the patients had chronic viral hepatitis. Only one patient (6%) had stage III HCC, 8 patients (44%) had stage IVA, and 9 patients (50%) stage IVB. Before the combined treatment, 12 patients (67%) received other treatments. All of them received local treatment to liver, including surgical resection, radiofrequency ablation, transarterial chemoembolization and RT. Two patients (11%) received systemic chemotherapy.
In the primary group, 12 of the 13 patients received RT using 3-dimensional conformal RT (3D CRT). For the remaining patient, RT was targeted to the liver lesion, metastatic lesions and distant lymph nodes, and treatment was performed by helical tomotherapy. The total radiation dose ranged from 30 to 54 Gy (median, 45 Gy).
In the measurable metastasis group, there were lymph node metastases in 3 patients, seeding nodules in the pelvic cavity in 1 patient, and a chest wall mass in 1 patient. Two patients with lymph node metastasis received 58.42 Gy with a daily dose of 2.54 Gy using helical tomotherapy. One patient with lymph node metastasis treated with 3D CRT received 50.4 Gy with 1.8 Gy daily. One patient with seeding nodules in the pelvic cavity received 39 Gy with 3 Gy daily using 3D CRT. Chest wall metastasis was treated with a total dose of 30 Gy in 6 Gy daily fractions with helical tomotherapy.
In patients treated with helical tomotherapy, the mean dose to the remaining liver volume, which is defined as the volume of the whole liver minus intrahepatic clinical target volume, was restricted to below 28 Gy, and the dose to the 2 cc of the duodenum was limited to 45 Gy.
Biologically effective dose (BED) was converted to conventional fractionation schedule of each dose due to variation in dose fractionation schedule. The α/β ratio was 10. After the conversion, the median dose of the primary group and the measurable metastasis group were 45 Gy and 50.4 Gy, respectively. Table 2 summarizes dose fractionation schedules of groups.
Sorafenib was administered twice a day with a total daily dose of 800 mg to 12 of 13 patients in the primary group, and 3 of 5 patients in the measurable metastasis group. The rest of the patients were treated with total daily dose of 400 mg because of their recent history of systemic chemotherapy prior to sorafenib or low baseline blood cell count. Sorafenib was administered concurrently with RT within one week of initial RT.
In the primary group, treatment response and progression were evaluated 1 month after the completion of RT using the modified Response Evaluation Criteria in Solid Tumors (RECIST).21 ORR was defined as partial response (PR) or complete response (CR). The level of serum α-fetoprotein (AFP) was checked before and after the combined treatment.
In the measurable metastasis group, their responses were evaluated between 1 and 3 months after the completion of RT using RECIST version 1.1.22 Criteria for determination of response were the same as above with a different definition for target lesions, which indicated whole lesions, but not contrast-enhancing lesions.
Overall survival (OS) rate and time to progression (TTP) were calculated from the day of RT initiation to the date of death and the date of progression, respectively, and were estimated by the Kaplan-Meier method.
According to the Common Terminology Criteria for Adverse Events v3.0, the development of toxicity was monitored through physical examination and laboratory testing for the levels of blood cell counts and chemistry, both during treatment and after the completion of treatment.
And, we divided patients according to the experience of dermatological adverse events during the RT including hand-foot skin reaction, erythema, and alopecia. The potential association between dermatological adverse events and efficacy was explored by comparing treatment response, OS, and TTP using chi-square test and log-rank test.
Tumor response was evaluated by imaging studies in 11 patients in the primary group and 5 patients in the measurable metastasis group. Two patients in the primary group who did not undergo imaging study after the treatment underwent follow-up of tumor markers only.
In the primary group, the size of target lesions was larger than 5 cm in all patients. The ORR of in-field target lesions by modified RECIST was 100% (Table 3). The mean value of size reduction rate for in-field target lesions was 61%. One patient showed CR of the target lesion inside of the radiation field, but out-field progression was observed on the 1-month follow-up imaging study (Fig. 1). Two patients showed lung metastasis on the 1-month follow-up CT. CT images of one patient are shown in Fig. 2. The mean serum AFP showed a decrease from the pre-treatment level of 8323±16837 IU/mL to the post-treatment level of 1501±2606 IU/mL. A decrease in AFP of more than 80% was observed in 7 patients (54%).
In patients who received RT for measurable metastasis, the ORR was 60%. Four patients (80%) showed a size reduction of the target lesions. Two patients (40%) had out-field progression on the 1-month follow-up imaging studies.
The median OS of all 18 patients was 7.8 months [95% confidence interval (CI), 3.0-12.6] and the 1 year-OS rate was 37%.
The median OSs of the primary group and the measurable metastasis group were 7.8 months (95% CI, 6.1-9.5) and 15.7 months (95% CI, 0-33.7), respectively. The 1 year-OS rates of the primary group and the measurable metastasis group were 35% and 60%, respectively.
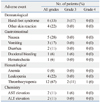
The incidence and proportion of grades 3 and 4 adverse events are listed in Table 4. The most common toxicity was thrombocytopenia. Two patients showed grade 3 thrombocytopenia, and 1 patient showed grade 4 thrombocytopenia. One of the two patients who showed grade 3 thrombocytopenia experienced gastrointestinal bleeding. The patient with grade 4 thrombocytopenia suffered from grade 3 duodenal hemorrhage requiring an endoscopic vigorous diagnostic approach. Due to the bleeding, the patient had to skip planned RT for a week.
The second most common toxicity was hand-foot syndrome. Three patients experienced grade 3 hand-foot syndrome, and another 2 patients complained of scrotal hair loss, desquamation, and eczema. After dose reduction of sorafenib, the symptoms were resolved. There was no interruption of treatment due to adverse events affecting the skin.
In one patient, liver enzyme aspartate aminotransferase (AST) level was elevated to 215 IU/L, which was a grade 3 side effect. The patient was asymptomatic, and the AST level was normalized after a week of conservative care. Overall, no treatment-related mortality was observed.
Most patients tolerated the treatment well and were able to complete the planned treatment. Adverse events at post-treatment 3 months and 6 months were checked to determine the occurrence of mid- and long-term side effects. There were 4 cases of side effects that occurred 3 months after the treatment. In the primary group, 2 patients experienced grade 1 radiation pneumonitis. Both of them had received RT to the lesions in the liver dome. One patient experienced grade 1 gastrointestinal discomfort because of sorafenib. The patient who had received RT to the pelvic cavity experienced grade 2 radiation proctitis. No side effects from the combined treatment occurred 6 months after the completion of the combined treatment.
Seven patients experienced dermatological adverse events and another 7 patients did not present any cutaneous symptom during the RT. All 7 patients (100%) with dermatological adverse events showed PR, while 4 of the 7 patients (57%) without the events gained PR (p=0.051). The median OS was 7.8 months (95% CI, 7.2-8.4) in the patients with dermatologic adverse events, versus 16.4 months (95% CI, 0-38.5) in the patients who did not develop the events (p=0.142). In terms of TTP, the results were 4.1 months (95% CI, 2.1-6.1) versus 6.9 months (95% CI, 6.5-7.3), without statistical significance (p=0.127).
In this work, we studied adverse events and treatment responses of combined treatment of sorafenib and RT in 18 patients. The ORR was 100% for the primary group and 60% for the measurable metastasis group. The most common adverse event was thrombocytopenia. Two patients experienced gastrointestinal hemorrhage without treatment-related mortality.
In randomized trials of sorafenib monotherapy, the incidences of overall and grade 3-4 thrombocytopenia were 46% and 4%, respectively, in the sorafenib group.14 Trials of RT on hepatic lesions combined with systemic or regional chemotherapeutic agents reported thrombocytopenia in 18-49% of patients and grade 3-4 thrombocytopenia in 6-12% of patients.23-25 In our present study, the incidence of overall thrombocytopenia was 67%, and that of grades 3-4 was 17%. The slightly higher incidence rate in our study might be attributed to two factors: concurrent administration of sorafenib with RT and a history of previous systemic chemotherapy. Two of the 3 patients who experienced grades 3-4 thrombocytopenia received systemic chemotherapy before undergoing the combined treatment. The myelosuppressive effect of previous chemotherapy might have contributed to the higher incidence of thrombocytopenia, although the overall incidence of hematologic toxicities was within the acceptable range. Further studies are needed to investigate underlying mechanism of this phenomenon.
In the present study, two patients experienced gastrointestinal hemorrhage; one patient with grade 3 duodenal bleeding presented as melena and another patient with rectal bleeding presented as hematochezia.
A patient with grade 3 duodenal bleeding, who also presented with grade 4 thrombocytopenia, had received 3 cycles of systemic 5-fluorouracil and carboplatin before the combination treatment. Her baseline platelet count before the combination treatment was 38000/µL and she received a reduced dose of sorafenib. Abdominal lymph node area was the radiation target, and she received 58.42 Gy with helical tomotherapy. After resting for one week with only conservative care, her duodenitis and melena were resolved, and she was able to complete the planned treatment. In the literature, the overall incidence of acute upper gastrointestinal bleeding has been reported as 2-38% after RT for liver tumors, associated with inclusion of the whole circumference of the hollow viscus in RT field, high radiation dose, and decreased hepatic function.26-28 In this patient, hepatic function was well preserved (Child-Pugh class A). Organs at risk were individually delineated and avoided as much as possible. Low platelet count as well as radiation enteritis might have contributed to duodenal bleeding, but the impact of sorafenib on the upper gastrointestinal bleeding needs to be evaluated in a larger number of patients. According to a report on the dose-response relationship by Park, et al.,29 the incidence of gastrointestinal complication in patients receiving the same dose as our patient did (>50 Gy) can be estimated as 13.2%.
In the patient with hematochezia, the radiation field was the pelvic cavity including seeding nodules as well as the rectum, and the total dose was 39 Gy in 3 Gy daily fractionations, which was compatible to 48.6 Gy (α/β=3) in a conventional BED. The patient presented with grade 2 diarrhea and hematochezia. Mild radiation proctitis was confirmed by colonoscopy. Grade 3 thrombocytopenia accompanied without a history of previous chemotherapy. After supportive care for a week, the patient recovered and completed the treatment. Radiation-induced lower gastrointestinal complication has been thoroughly investigated in an acute radiation proctitis model, occurring in 26.4-47% of patients after receiving pelvic irradiation.30-33 Increased mean rectal dose and irradiated rectal volume are predictive factors for rectal bleeding.32,33
These two patients have 2 factors in common: treatment-associated grade 3 or 4 thrombocytopenia and RT field with close proximity to the gastrointestinal tract. In patients harboring target lesions close to the gastrointestinal tract, more attention is required to precisely delineate the tumor and surrounding normal organs when planning RT, in order to avoid overdosing the gastrointestinal tract.
There was no radiation-induced hepatic toxicity in our study, except for 1 patient who presented with transient AST elevation without any symptoms. Considering that 11 of 13 patients in the primary group (85%) had viral etiology and the range of total dose was 30-54 Gy, the low incidence of hepatic toxicity observed in this study signifies that RT combined with sorafenib could be safely applied to hepatic lesions.
We applied the modified RECIST to the primary group because the target lesions were hepatic masses. To our surprise, the in-field treatment response rate was 100% in the primary group by modified RECIST, while 27% was derived by RECIST 1.1 (Table 3). Thus, the modified RECIST appears to provide more useful prognostic information than the RECIST, especially in HCC patients who received anti-angiogenic agents.21 Consequently, this excellent response rate might have led to a better OS of our patients than that of a previous report.13 The causal relationship between the excellent treatment response and better survival should be proven in future studies.
We analyzed the relationship between dermatological adverse events during RT and the treatment outcomes. Previous study on the association of early skin toxicity and the tumor control in HCC patients treated with sorafenib showed that patients with at least grade 1 skin toxicity had better tumor control rate as well as longer TTP.34 Despite better tumor response of the patients with dermatological toxicities in our study, it did not extend to better TTP and OS. However, it is necessary to evaluate potential predictive factors for treatment outcome in these patients.
Our study is limited by a small number of patients and a retrospective nature of the study. Despite such limitations, this study shows that the combined treatment of sorafenib and RT is safe and effective in advanced HCC. The outcome observed in this study can contribute to the future clinical studies of combining sorafenib and RT. At present, a phase I study of sorafenib and RT in a concurrent manner is ongoing (ClinicalTrials.gov number, NCT00892658), as well as another study in a sequential manner (ClinicalTrials. gov number, NCT00999843).
In the combined treatment of sorafenib with RT, acute toxicities were manageable, no treatment-related mortality was observed, and a substantial tumor response was achieved in the target lesions. Despite its limitations, our experience emphasizes the importance of a multidisciplinary approaches and individualized medicine in the management of advanced HCC. Further elucidation of the mechanisms of interaction between sorafenib and RT is also warranted.
Figures and Tables
Fig. 1
Illustrations of a patient in the primary group who achieved in-field CR but had out-field progression in the liver. (A) The pretreatment computed tomography (CT) scan shows two lesions. The lesion located in the segment 6 was treated with transarterial chemoembolization. (B) Axial dose distribution of 3-D conformal radiotherapy (RT). The lesion in the segment 4 was treated with RT, and (C) 1 month after completion of RT, a follow-up CT scan shows disappearance of the lesion in the segment 4. Unfortunately, the lesion in the segment 6 had progressed. CR, complete remission.
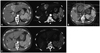
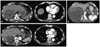
Fig. 2
Illustrations of a patient in the primary group who achieved in-field PR but showed progression of lung metastasis. (A) The pretreatment computed tomography (CT) scan shows a huge mass invading the right portal vein. Small metastatic nodules are seen in both lungs (arrows). (B) Axial dose distribution of 3-D conformal radiotherapy (RT), and (C) 1 month after completion of RT, a follow-up abdomen CT scan shows a decrease in size in the target lesion, but a chest CT scan shows new metastatic nodules in both lungs (arrows). PR, partial response.
ACKNOWLEDGEMENTS
This work was supported by the National R&D program grant for cancer control, the Ministry of Health and Welfare (0620390).
Notes
References
1. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007; 132:2557–2576.

2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.

3. Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999; 19:271–285.

4. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.

5. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004; 127:5 Suppl 1. S35–S50.

6. Park KW, Park JW, Choi JI, Kim TH, Kim SH, Park HS, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2008; 23:467–473.

7. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004; 64:7099–7109.

8. Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007; 59:561–574.

9. Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, et al. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998; 27:951–958.

10. Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007; 27:55–76.

11. Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006; 130:1117–1128.

13. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.

14. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.

15. Zhang T, Ding X, Wei D, Cheng P, Su X, Liu H, et al. Sorafenib improves the survival of patients with advanced hepatocellular carcinoma: a meta-analysis of randomized trials. Anticancer Drugs. 2010; 21:326–332.

16. National Comprehensive Cancer Network. Hepatobiliary cancers, version 2. accessed on 2012 July 10. Available at http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.
17. Han KH, Seong J, Kim JK, Ahn SH, Lee do Y, Chon CY. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis. Cancer. 2008; 113:995–1003.

18. Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. 2005; 25:1189–1196.

19. Korean Liver Cancer Study Group and National Cancer Center, Korea. [Practice guidelines for management of hepatocellular carcinoma 2009]. Korean J Hepatol. 2009; 15:391–423.
20. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003; 38:207–215.

21. Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, et al. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012; 118:147–156.

22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.

23. Robertson JM, McGinn CJ, Walker S, Marx MV, Kessler ML, Ensminger WD, et al. A phase I trial of hepatic arterial bromodeoxyuridine and conformal radiation therapy for patients with primary hepatobiliary cancers or colorectal liver metastases. Int J Radiat Oncol Biol Phys. 1997; 39:1087–1092.

24. Stillwagon GB, Order SE, Guse C, Klein JL, Leichner PK, Leibel SA, et al. 194 hepatocellular cancers treated by radiation and chemotherapy combinations: toxicity and response: a Radiation Therapy Oncology Group Study. Int J Radiat Oncol Biol Phys. 1989; 17:1223–1229.

25. Mohiuddin M, Chen E, Ahmad N. Combined liver radiation and chemotherapy for palliation of hepatic metastases from colorectal cancer. J Clin Oncol. 1996; 14:722–728.

26. Yoon SM, Kim JH, Choi EK, Ahn SD, Lee SW, Yi BY, et al. Radioresponse of hepatocellular carcinoma-treatment of lymph node metastasis. Cancer Res Treat. 2004; 36:79–84.

27. Zhou ZH, Liu LM, Chen WW, Men ZQ, Lin JH, Chen Z, et al. Combined therapy of transcatheter arterial chemoembolisation and three-dimensional conformal radiotherapy for hepatocellular carcinoma. Br J Radiol. 2007; 80:194–201.

28. Cheng JC, Chuang VP, Cheng SH, Huang AT, Lin YM, Cheng TI, et al. Local radiotherapy with or without transcatheter arterial chemoembolization for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2000; 47:435–442.

29. Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002; 54:150–155.

30. Wang CJ, Leung SW, Chen HC, Sun LM, Fang FM, Huang EY, et al. The correlation of acute toxicity and late rectal injury in radiotherapy for cervical carcinoma: evidence suggestive of consequential late effect (CQLE). Int J Radiat Oncol Biol Phys. 1998; 40:85–91.

31. Sanguineti G, Endres EJ, Parker BC, Bicquart C, Little M, Chen G, et al. Acute toxicity of whole-pelvis IMRT in 87 patients with localized prostate cancer. Acta Oncol. 2008; 47:301–310.

32. Peeters ST, Heemsbergen WD, van Putten WL, Slot A, Tabak H, Mens JW, et al. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys. 2005; 61:1019–1034.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download