Abstract
Purpose
NAD(P)H:Quinone Oxidoreductase 1 (NQO1) C609T missense variant (NQO1*2) and 29 basepair (bp)-insertion/deletion (I29/D) polymorphism of the NRH:Quinone Oxidoreductase 2 (NQO2) gene promoter have been proposed as predictive and prognostic factors for cancer development and progression. The purpose of this study is to investigate the relationship between NQO1/NQO2 genotype and clinico-pathological features of papillary thyroid microcarcinoma (PTMC).
Materials and Methods
Genomic DNA was isolated from 243 patients; and clinical data were retrospectively analyzed. NQO1*2 and tri-allelic polymorphism of NQO2 were investigated by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis.
Results
PTMC with NQO1*2 frequently exhibited extra-thyroidal extension as compared to PTMC with wild-type NQO1 (p=0.039). There was a significant relationship between I29/I29 homozygosity of NQO2 and lymph node metastasis (p=0.042). Multivariate analysis showed that the I29/I29 genotype was associated with an increased risk of lymph node metastasis (OR, 2.24; 95% CI, 1.10-4.56; p=0.026).
NAD(P)H:Quinone Oxidoreductase 1 (NQO1) and NRH:Quinone Oxidoreductase 2 (NQO2) have been reported to confer protection against oxidative stress and carcinogenesis.1-3 Homozygous deletion of NQO1 in mice results in reduced p53 induction and decreased apoptosis; these knockout mice also exhibit an increased susceptibility to chemical-induced skin carcinogenesis.4 In NQO2-null mice, p53 is rapidly degraded and B-cell apoptosis is decreased, which suggests that NQO2 functions similarly to NQO1.5
Recent genetic studies have revealed that polymorphisms of NQO1 and NQO2 can alter enzymatic activity and cancer susceptibility. A well-characterized polymorphism of NQO1 is NQO1*2, a C609T missense variant (rs1800566, NP-000894:p.187P>S) that results in proline-187-serine (P187S) substitution of NQO1.6 NQO1 P187S is rapidly degraded through the ubiquitin proteosomal pathway, and NQO1*2 carriers have decreased NQO1 activity.7 NQO1*2 is also associated with an increased risk of developing cancer and poor prognosis in leukemia and breast cancer patients.8,9 The most widely studied NQO2 polymorphism is a 29 basepair (bp)-insertion/deletion (I29/D) polymorphism of the NQO2 gene promoter.10 This tri-allelic polymorphism consists of a 29 bp-insertion (I29), a 29 bp-deletion (D), and a 16 bp-insertion (I16). Recent data from genetic studies suggest that Sp3, a repressor of NQO2 gene expression, binds to I29 in the NQO2 promoter, resulting in decreased gene expression and enzymatic activity of NQO2.11 Consistent with this observation, it has been suggested that the I29 allele may be a candidate cancer susceptible gene in breast cancer.12
Papillary thyroid cancer (PTC) is the most common endocrine cancers, andthe incidence of PTC has been increasing over the last decade.13 Well-characterized risk factors for PTC are radiation exposure and iodine,14 both of which can generate reactive oxygen species (ROS) that induce oxidative stress and directly damage the DNA in follicular cells.15 Indeed, oxidative DNA damage increases the spontaneous mutation rate (SMR) in mouse follicular cells.16 These results suggest that oxidative stress induced by certain carcinogens may be a causative factor in the initiation or progression of PTC, and enzymes that serve to link antioxidant systems, such as NQO1 and NQO2, might have a protective role against thyroid carcinogenesis.
Studies into a genetic predisposition to thyroid cancer have suggested that forkhead box E1 and NK2 homeobox 1 are potential susceptibility loci in thyroid cancer;17 however, similar studies to define susceptible genes for oxidative stress have not been successful. Furthermore, it is not known whether there is a relationship between oncogenic BRAFV600E and susceptibility genes for oxidative stress, as has been shown for other cancers. For example, in colorectal cancer, the NQO1*2 polymorphism modified cancer risk and was significantly associated with the K-ras codon 12 mutation.18
There is another presumptive evidence for a protective role of NQO1 and NQO2 in thyroid carcinogenesis; these proteins can stabilize and increase p53 levels. The presence of an inactivating mutation of p53 is relatively rare in differentiated thyroid cancer, suggesting that alternative mechanisms, such as functional inactivation of p53, may be involved in the initiation or progression of PTC.19 Decreased expression of NQO1 and NQO2 could represent one such mechanism of permissive carcinogenesis in PTC.
In the present study, we investigated the frequency and clinical outcomes associated with the NQO1*2 polymorphism and the tri-allelic polymorphism of the NQO2 promoter in nodular hyperplasia (NH) and papillary thyroid microcarcinoma (PTMC), in order to elucidate the genotype-phenotype relationship between these polymorphisms and clinico-pathological features of PTMC.
This is a retrospective study of 243 patients who underwent thyroid surgery because of their incidentally discovered thyroid nodules between June, 2002, and December, 2006, at the Center for Endocrine Surgery, Chungnam National University Hospital and St. Mary's Hospital, The Catholic University of Korea. Under the American Thyroid Association guidelines, US-guided fine needle aspiration biopsy (FNAB) was performed for nodules smaller than 1 cm which showed suspicious ultrasound findings. On the basis of FNAB cytological analysis, patient with malignant and indeterminate or suspicious neoplasm underwent thyroid surgery and final pathologic diagnosis was obtained. Genomic DNA from paraffin-embedded tissue was isolated using the QIAamp DNA Mini Kit (Qiagen, Chatsworth, CA, USA). The Institutional Review Boards of each university approved all of the study protocols.
Exon 15 of the BRAF gene was amplified from genomic DNA by PCR (5 µL reaction volume) and then sequenced by pyrosequencing, as previously described.20 In brief, DNA amplification was performed using the following primers: forward, 5'-ATGCTTGCTCTGATAGGAAA-3', and reverse, 5'-ATTTTTGTGAATACTGGGGAA-3'. Amplified products were immobilized on magnetic beads, and then the bead/DNA complexes were subjected to pyrosequencing with the forward primer 5'-GGTGATTTTGGTCTAGCTAC-3'. Final single-stranded DNA templates were transported into a PSQ HS 96 A pyrosequencer system (Biotage, Uppsala, Sweden).
The substitution of C to T in exon 3 of NQO1 was detected by PCR-restriction fragment length polymorphism (RFLP) analysis using HinfI, as previously described.21 Briefly, genomic DNA was amplified in a 5 µL reaction volume by PCR using the following primers: forward, 5'-AGTGGCATTCTGCATTTCTGTG-3', and reverse, 5'-GATGGACTTGCCCAAGTGATG-3'. Following restriction enzyme digestion by HinfI, wild-type NQO1 (NQO1 CC) appeared as two bands of 189 bp and 85 bp. Homozygous NQO1*2 (NQO1 TT) appeared as three bands (151 bp, 85 bp and 37 bp), and heterozygous NQO1*2 (NQO1 CT) appeared as bands of all four sizes.
The I29/D polymorphism was detected by PCR-RFLP analysis using the following primers: forward, 5'-CTGCCTGGAAGTCAGCAGGGTC-3', and reverse, 5-CTCTTTACGCAGCGCGCCTAC-3'.22 The theoretical molecular weight of the amplified I29 allele was 290 bp, while that of I16 was 277 bp and D was 261 bp. Since agarose gel electrophoresis was insufficient to discriminate between I29 and I16, RFLP analysis was carried out using Ava I, which yielded 250 bp and 40 bp I29 fragments.
Immunohistochemistry in normal thyroid tissue, NH and PTMC was performed. Briefly, 4 µm thick tissue sections were heated at 60℃, deparaffinized in xylene, and then hydrated in a graded series of alcohol. Then, antigen retrieval was performed by microwaving the samples in citrate buffer for 10 min. Endogenous peroxidase activity was inactivated by incubation in a solution of 3% hydrogen peroxide for 10 min, and then nonspecific binding sites were blocked by incubation in 10% normal goat serum in phosphate-buffered saline (PBS). Tissue sections were incubated with NQO1 (Cell Signaling Technology, Boston, MA, USA) or nuclear factor (erythroid-derived 2)-like 2 (NFE2L2 or Nrf2, Abcam plc, Cambridge, UK) primary antibody for 60 min at room temperature, after which they were treated sequentially with biotinylated anti-mouse immunoglobulin for 30 min, peroxidase-labeled streptavidin for 30 min, and diaminobenzidine in the presence of hydrogen peroxide. As a control, samples were incubated with PBS instead of primary antibody. No positive staining was observed in the control samples. Tissue sections of colon and breast carcinoma were also analyzed as positive controls.
Hardy-Weinberg equilibrium and group comparisons of categorical variables were examined by the chi-square test or linear by linear association. Comparisons of means were evaluated with the independent samples t-test. Multivariate logistic regression analysis was performed to assess the association of NQO1*2 with extra-thyroidal extension and the I29/I29 genotype with lymph node metastasis. Confidence intervals (CIs) were computed by standard methods. All reported p-values are two sided and all analyses were performed using SPSS Versions 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
Increased expression of NQO1 has frequently been documented in developing tumors, which suggests that NQO1 participates in cellular defense mechanisms that protect against carcinogenesis.23 To validate these previous results in thyroid carcinoma, normal thyroid, NH and PTMC tissues were subjected to immunohistochemistry using an anti-NQO1 antibody. As shown in Fig. 1A, NQO1 was not readily detected in normal thyroid follicles. However, focal expression of NQO1 was observed in normal follicular cells and NH, particularly in the apical areas of follicular cells where relatively large amounts of H2O2 are generated for the synthesis of thyroid hormone (Fig. 1B and C). These results suggested that oxidative stress induces NQO1 expression in follicular cells. Diffuse cytoplasmic staining was readily observed in the PTMC harboring wild type NQO1 (Fig. 1D and E), although in that of patients carrying an NQO1*2 polymorphism, the intensity of NQO1-positive signals was reduced (Fig. 1F). It is apparent that the expression of wild type NQO1 levels increased in cancer cells as compared to normal follicular cells, perhaps due to increased oxidative stress in these cells. Furthermore, patients with the NQO1*2 polymorphism appeared to be defective in oxidative stress-induced expression of NQO1. Taken together, it is highly likely that NQO1*2 is linked to cancer initiation or progression in PTMC.
To better understand the role of NQO1 in PTMC, we investigated the frequency of the NQO1*2 polymorphism in PTMC. Of 173 patients (48 NH and 125 PTMC) selected for the analysis (Table 1), none of the samples was homozygous for the NQO1*2 allele; however, the minor allele frequencies was 0.29, which was in Hardy-Weinberg equilibrium (p=0.926). The frequency of heterozygous NQO1*2 in NH was 58.3% (28/48) and 56.8% (71/125) in PTMC. There was no significant difference in the frequency of NQO1*2 polymorphism between the two groups.
Immunohistochemical analysis of NQO2 expression in NH and PTMC was not possible due to unavailability of anti-NQO2 antibody. However, since NQO2 also functions in protecting cells against oxidative stress, we examined the frequency of the I29/D polymorphism of the NQO2 gene promoter in patients with PTMC. Seventy-three NH and 170 PTMC were analyzed, and the results are summarized in Table 1. In NH, 54.8% (40/73) of the samples were homozygous for the I29 allele (I29/I29), 31.5% (23/73) were I29/D heterozygotes, and 13.7% (10/73) were homozygous for the D allele (D/D). The frequencies of these NQO2 gene promoter polymorphisms were similar to those of PTMC, in which 51.8% (88/170) of the samples were of the I29/I29 genotype, 32.9% (56/170) were I29/D heterozygotes, and 14.2% (24/170) were D/D homozygotes. The minor allele frequency was 0.30, which was in Hardy-Weinberg equilibrium (p=0.947). We identified two PTMC samples that were of the I29/I16 genotype, but these samples were excluded from the analysis because the frequency of this genotype was too low to determine clinical significance.
Clinico-pathological parameters such as age at diagnosis, gender, tumor size, capsular invasion, multifocality, bilaterality, lymph node metastasis and the presence of BRAFV600E mutation were similar in the presence and absence of NQO1*2 polymorphism. The presence of extra-thyroidal extension, however, was more common in PTMCs harboring NQO1*2 as compared to wild-type NQO1 (p=0.039) (Table 2). Multivariate analysis of the relationship between NQO1*2 and extra-thyroidal extension was carried out, omitting capsular invasion and TNM stage, as these factors are closely related to extra-thyroidal extension of PTMC. The results revealed no clear relationship between NQO1*2 and extra-thyroidal extension (odds ratio, 1.82; 95% CI, 0.85-3.89; p=0.124) (Table 3).
Analysis of the I29/D polymorphism revealed that 43% of patients with the I29/I29 genotype experienced lymph node metastasis as compared to only 20% of patients with the D/D genotype. This association between I29 homozygosity and lymph node metastasis was statistically significant (p=0.042) (Table 4). Furthermore, multivariate analysis clearly showed a significant correlation between the I29 allele and lymph node metastasis (odds ratio, 2.24; 95% CI, 1.10-4.56; p=0.026) (Table 5).
To investigate the different anti-oxidative response according to the presence or absence of NQO1*2 and NQO2 I29, we performed immunohistochemical staining for Nrf2. Nrf2 is a well-known transcription activator binding to antioxidant response elements and can be a useful marker against oxidative stress.24 As shown in Fig. 2, PTMC harboring NQO1*2 and NQO2 I29 showed highest staining intensity of Nrf2, suggesting that the absence of NQO1 and NQO2 might provoke strong oxidative response.
In the present study, we examined the frequency of the NQO1*2 and I29/D polymorphisms of NQO1 and NQO2, respectively, in NH and PTMC. Although NQO1*2 has been suggested to be a susceptibility polymorphism in lung cancer, leukemia and urogenital malignancy,3,4,25,26 our genetic analysis did not reveal an increase in the frequency of NQO1*2 in PTMC as compared to that in NH. In addition, analysis of the frequency of NQO2 promoter polymorphisms in PTMC and NH also revealed no significant differences in the frequency of the I29/D polymorphisms. However, the specific function of the thyroid gland is to produce a large amount of H2O2 for thyroid hormone synthesis; therefore, follicular cells are likely to be highly susceptible to oxidative damage and vulnerable to frequent mutagenesis, particularly if antioxidant systems in these cells are not properly functioning.27-29 Animal experiments have shown that epithelial cells around the follicular lumen exhibit an increased SMR in the thyroid gland.27 In the current study, NQO1 expression was detected by immunohistochemistry in normal follicular cells and NH, particularly in apical areas, suggesting that NQO1 is involved in the cellular response to physiological or pathological oxidative stress. Thus, NQO1*2 may represent a susceptibility polymorphism for various thyroid diseases accompanied by excessive ROS production.30 Although we were unable to perform immunohistochemistry of NQO2, we presume that the function of NQO2 in the thyroid is most likely similar to that of NQO1.
Anti-oxidant enzymes such as thioredoxin reductase and glutathione peroxidase 3 (GPX3) have been proposed as protective mechanisms against H2O2-induced oxidative stress and are potentially involved in the regulation of thyroid hormone synthesis. There is a clear association between GPX3 gene polymorphism and the occurrence of differentiated thyroid cancer, which is consistent with an important role in antioxidant systems of protecting cells against DNA mutation and carcinogenesis.31 To definitively confirm the protective effect of NQO1 and NQO2 against mutagenesis, such as in BRAFV600E-induced carcinoma, a more comprehensive analysis of other genes that are coordinately induced with NQO1, such as glutathione S-transferase, UDP-glucuronyl transferase, and γ-glutamylcysteine synthase, should also be performed. Also, it is important to note that the current study did not analyze other known polymorphisms of NQO2, such as rs2071002 (+237A>C), which has recently been identified as a risk allele in breast cancer.12 Emerging polymorphisms should be included in future studies of the roles of NQO1 and NQO2 in defending against ROS-induced mutagenesis. However, in our immunohistochemistry study for Nrf2, the presence of NQO1*2 and NQO2 I29 strongly induced Nrf2 expression, suggesting that dysfunction of NQO1 and NQO2 might be able to provoke higher oxidative response in tumor cells.
In general, most of studies of NQO1 and NQO2 polymorphisms have used genomic DNA isolated from blood. In the current study, however, we used DNA from tumor because this study was designed retrospectively. Activating mutations such as H-RASV16 or BRAFV600E induces genomic instability through constitutive activation of mitogen-activated protein kinase, a mechanism that may induce additional somatic mutation.32 To determine whether NQO1 or NQO2 polymorphisms were due to genomic instability, NQO1 and NQO2 polymorphisms in genomic DNA from tumor and contralateral normal tissue from the same patient were analyzed in 45 PTMCs. However, there was no difference in polymorphism status between tumor tissue and contralateral normal tissue in any of the samples (data not shown), indicating that NQO1 and NQO2 polymorphisms in these tumors were not due to genomic instability.
In this study, PTMC with NQO1*2 polymorphism was frequently accompanied by extra-thyroidal extension, although multivariate analysis did not support a strong relationship. Perhaps, the most intriguing result of the current study was that the I29 allele exhibited a marked association with lymph node metastasis. Multivariate analysis clearly showed that patients with the I29/I29 genotype were at higher risk of lymph node metastasis. In fact, previous studies reported that the NQO1*2 is a prognostic marker for leukemia, breast and lung cancers to predict poor patients' outcome and resistance to conventional therapy.8,9,33 However, the prognostic impact of the I29/I29 genotype has not been extensively investigated, although the inactivation of NQO1 and NQO2 is strongly related to the multiplicity of skin tumors in murine models.34 The mechanism by which ROS can affect tumor behaviors is the genotoxicity of ROS such as ROS-mediated DNA damage, ineffective DNA repair mechanisms or genomic instability.35,36 The other important ROS effect on tumor cells is mitochondrial DNA alterations because mitochondrial DNA has no histone protection.37 Epigenetic alteration such as aberrant CpG island hypermethylation is also related to ROS-mediated carcinogenesis.38,39 To the best of our knowledge, we could not define the exact protective mechanism of NQO1 and NQO2 against carcinogenesis. However, we suspect that excessive accumulation of ROS is able to promote cancer initiation, promotion and progression, and that the defect of cellular defense system such as NQO1*2 and the I29/I29 genotype may aggravate genetic and epigenetic alteration in human carcinogenesis.
Taken together, these clinical features associated with NQO1*2 and I29 alleles in PTMC suggested that NQO2 polymorphisms may significantly affect enzymatic activities and attenuate the preventive functions of NQO2 against cancer progression. Thus, I29 allele represents a putative prognostic indicator for PTMC.
Analysis of frequency and clinical impact of NQO1*2 and I29 allele polymorphisms of NQO1 and NQO2, respectively, revealed that patients who carry the NQO1*2 polymorphism might be predisposed to extra-thyroidal extension of PTMC, while the I29 allele of NQO2 was strongly associated with lymph node metastasis. Future studies on the biological role and the prognostic indicator of these NQO1 and NQO2 polymorphisms will contribute to a better understanding of the role of oxidative stress defense mechanisms in the development and progression of PTMC.
Figures and Tables
Fig. 1
Immunohistochemical staining of NQO1 in the normal thyroid and PTMC. (A) NQO1 was rarely detected in normal thyroid follicles. (B and C) Focal expression of NQO1 was observed in the apical areas of normal follicular cells (boxed area and arrows). (D and E) Diffuse intense cytoplasmic staining was observed in papillary thyroid cancer cells from same patient with wild-type NQO1. (F) NQO1 expression was barely detectable in a patient with heterozygous NQO1*2 polymorphism. All results are representative images. NQO1, NAD(P)H:Quinone Oxidoreductase 1; PTMC, papillary thyroid microcarcinoma.
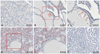
Fig. 2
Representative immunohistochemical staining of Nrf2. (A) Nrf2 was rarely detected in PTMC with wild type NQO1 and NQO2. (B and C) Moderate expression of Nrf2 was observed in PTMC with NQO1*2 (B) or NQO2 I29/I29 (C). (D) Strong and diffuse intense staining was observed in PTMC with NQO1*2 and NQO2 I29/I29. PTMC, papillary thyroid microcarcinoma; NQO1, NAD(P)H:Quinone Oxidoreductase 1; NQO2, NRH:Quinone Oxidoreductase 2.

Table 1
Frequency of NQO1 and NQO2 Polymorphisms in Nodular Hyperplasia and Papillary Thyroid Microcarcinoma

NQO1, NAD(P)H:Quinone Oxidoreductase 1; NQO2, NRH:Quinone Oxidoreductase 2; NH, nodular hyperplasia; PTMC, papillary thyroid microcarcinoma. C/C: wild type NQO1. C/T: heterozygote C609T missense variant of NQO1. I29: 29 basepair insertion polymorphism of NQO2. D: 29 basepair deletion polymorphism of NQO2.
*The NQO1 polymorphisms of 25 cases of 73 NH and 45 cases of 170 PTMC were not able to be assessed.
†Two cases of PTMC showing the I29/I16 genotype were not included.
‡Pair-wise comparisons from the Pearson χ2 test.
§Comparisons of three or four groups using linear by linear association.
Table 3
Multivariate Analyses of the Association of Extra-Thyroidal Extension with NQO1*2 in Patients with PTMC

ACKNOWLEDGEMENTS
This work was supported in part by the second phase of the Brain Korea 21 program of the Ministry of Education and NRF/MEST (No. 2010-0005462, 2012R1A2A2A01014672).
References
1. Shaw PM, Reiss A, Adesnik M, Nebert DW, Schembri J, Jaiswal AK. The human dioxin-inducible NAD(P)H: quinone oxidoreductase cDNA-encoded protein expressed in COS-1 cells is identical to diaphorase 4. Eur J Biochem. 1991; 195:171–176.

2. Jaiswal AK, Burnett P, Adesnik M, McBride OW. Nucleotide and deduced amino acid sequence of a human cDNA (NQO2) corresponding to a second member of the NAD(P)H:quinone oxidoreductase gene family. Extensive polymorphism at the NQO2 gene locus on chromosome 6. Biochemistry. 1990; 29:1899–1906.

3. Long DJ 2nd, Jaiswal AK. Mouse NRH:quinone oxidoreductase (NQO2): cloning of cDNA and gene- and tissue-specific expression. Gene. 2000; 252:107–117.

4. Iskander K, Gaikwad A, Paquet M, Long DJ 2nd, Brayton C, Barrios R, et al. Lower induction of p53 and decreased apoptosis in NQO1-null mice lead to increased sensitivity to chemical-induced skin carcinogenesis. Cancer Res. 2005; 65:2054–2058.

5. Iskander K, Barrios RJ, Jaiswal AK. NRH:quinone oxidoreductase 2-deficient mice are highly susceptible to radiation-induced B-cell lymphomas. Clin Cancer Res. 2009; 15:1534–1542.

6. Ross D, Beall H, Traver RD, Siegel D, Phillips RM, Gibson NW. Bioactivation of quinones by DT-diaphorase, molecular, biochemical, and chemical studies. Oncol Res. 1994; 6:493–500.
7. Siegel D, Anwar A, Winski SL, Kepa JK, Zolman KL, Ross D. Rapid polyubiquitination and proteasomal degradation of a mutant form of NAD(P)H:quinone oxidoreductase 1. Mol Pharmacol. 2001; 59:263–268.

8. Rothman N, Smith MT, Hayes RB, Traver RD, Hoener B, Campleman S, et al. Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C-->T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res. 1997; 57:2839–2842.
9. Fagerholm R, Hofstetter B, Tommiska J, Aaltonen K, Vrtel R, Syrjäkoski K, et al. NAD(P)H:quinone oxidoreductase 1 NQO1*2 genotype (P187S) is a strong prognostic and predictive factor in breast cancer. Nat Genet. 2008; 40:844–853.

10. Wang W, Le WD, Pan T, Stringer JL, Jaiswal AK. Association of NRH:quinone oxidoreductase 2 gene promoter polymorphism with higher gene expression and increased susceptibility to Parkinson's disease. J Gerontol A Biol Sci Med Sci. 2008; 63:127–134.

11. Wang W, Jaiswal AK. Sp3 repression of polymorphic human NRH:quinone oxidoreductase 2 gene promoter. Free Radic Biol Med. 2004; 37:1231–1243.

12. Yu KD, Di GH, Yuan WT, Fan L, Wu J, Hu Z, et al. Functional polymorphisms, altered gene expression and genetic association link NRH:quinone oxidoreductase 2 to breast cancer with wild-type p53. Hum Mol Genet. 2009; 18:2502–2517.

13. Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009; 115:3801–3807.

15. Chen W, Man N, Shan Z, Teng W. Effects of long-term exposure to iodine excess on the apoptosis of thyrocytes in Wistar rats. Exp Clin Endocrinol Diabetes. 2011; 119:1–8.

16. Gossen JA, de Leeuw WJ, Molijn AC, Vijg J. Plasmid rescue from transgenic mouse DNA using LacI repressor protein conjugated to magnetic beads. Biotechniques. 1993; 14:624–629.
17. Kouniavsky G, Zeiger MA. Thyroid tumorigenesis and molecular markers in thyroid cancer. Curr Opin Oncol. 2010; 22:23–29.

18. Lafuente MJ, Casterad X, Trias M, Ascaso C, Molina R, Ballesta A, et al. NAD(P)H:quinone oxidoreductase-dependent risk for colorectal cancer and its association with the presence of K-ras mutations in tumors. Carcinogenesis. 2000; 21:1813–1819.

19. Farid NR. P53 mutations in thyroid carcinoma: tidings from an old foe. J Endocrinol Invest. 2001; 24:536–545.

20. Jo YS, Huang S, Kim YJ, Lee IS, Kim SS, Kim JR, et al. Diagnostic value of pyrosequencing for the BRAF V600E mutation in ultrasound-guided fine-needle aspiration biopsy samples of thyroid incidentalomas. Clin Endocrinol (Oxf). 2009; 70:139–144.

21. Kelsey KT, Ross D, Traver RD, Christiani DC, Zuo ZF, Spitz MR, et al. Ethnic variation in the prevalence of a common NAD(P)H quinone oxidoreductase polymorphism and its implications for anti-cancer chemotherapy. Br J Cancer. 1997; 76:852–854.

22. Yu KD, Di GH, Fan L, Hu Z, Chen AX, Shao ZM. Caution regarding genotyping methodology for a tri-allelic polymorphism in the novel breast cancer susceptibility gene NQO2. Breast Cancer Res Treat. 2009; 118:647–649.

23. Belinsky M, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993; 12:103–117.

24. Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004; 10:879–891.

25. Chao C, Zhang ZF, Berthiller J, Boffetta P, Hashibe M. NAD(P)H:quinone oxidoreductase 1 (NQO1) Pro187Ser polymorphism and the risk of lung, bladder, and colorectal cancers: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006; 15:979–987.

26. Long DJ 2nd, Gaikwad A, Multani A, Pathak S, Montgomery CA, Gonzalez FJ, et al. Disruption of the NAD(P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res. 2002; 62:3030–3036.
27. Maier J, van Steeg H, van Oostrom C, Karger S, Paschke R, Krohn K. Deoxyribonucleic acid damage and spontaneous mutagenesis in the thyroid gland of rats and mice. Endocrinology. 2006; 147:3391–3397.

28. Krause K, Karger S, Schierhorn A, Poncin S, Many MC, Fuhrer D. Proteomic profiling of cold thyroid nodules. Endocrinology. 2007; 148:1754–1763.

29. Krohn K, Maier J, Paschke R. Mechanisms of disease: hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors. Nat Clin Pract Endocrinol Metab. 2007; 3:713–720.

30. Siegel D, Ross D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med. 2000; 29:246–253.
31. Yan F, Yang WK, Li XY, Lin TT, Lun YN, Lin F, et al. A trifunctional enzyme with glutathione S-transferase, glutathione peroxidase and superoxide dismutase activity. Biochim Biophys Acta. 2008; 1780:869–872.

32. Saavedra HI, Knauf JA, Shirokawa JM, Wang J, Ouyang B, Elisei R, et al. The RAS oncogene induces genomic instability in thyroid PCCL3 cells via the MAPK pathway. Oncogene. 2000; 19:3948–3954.

33. Song SY, Jeong SY, Park HJ, Park SI, Kim DK, Kim YH, et al. Clinical significance of NQO1 C609T polymorphisms after postoperative radiation therapy in completely resected non-small cell lung cancer. Lung Cancer. 2010; 68:278–282.

34. Shen J, Barrios RJ, Jaiswal AK. Inactivation of the quinone oxidoreductases NQO1 and NQO2 strongly elevates the incidence and multiplicity of chemically induced skin tumors. Cancer Res. 2010; 70:1006–1014.

35. Francisco DC, Peddi P, Hair JM, Flood BA, Cecil AM, Kalogerinis PT, et al. Induction and processing of complex DNA damage in human breast cancer cells MCF-7 and nonmalignant MCF-10A cells. Free Radic Biol Med. 2008; 44:558–569.

36. Holt SM, Georgakilas AG. Detection of complex DNA damage in gamma-irradiated acute lymphoblastic leukemia Pre-b NALM-6 cells. Radiat Res. 2007; 168:527–534.

37. Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)--induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011; 711:167–173.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download