Abstract
Purpose
Late-presenting congenital diaphragmatic hernia (CDH) beyond the neonatal period is rare and often misdiagnosed, with delayed treatment.
Materials and Methods
We retrospectively reviewed our experience with late-presenting CDH over 30 years at a single institution to determine the characteristics of late-presenting CDH for early diagnosis.
Results
Seven patients had operations due to late-presenting CHD in our institution over 30 years. The patients' ages ranged from 2.5 months to 16 years. There were six boys and one girl. Five hernias were left-sided, one was right-sided and one was a retrosternal hernia. All patients had normal intestinal rotation. Non-specific gastrointestinal or respiratory symptoms and signs were usually presented. Intestinal malrotations were absent; therefore, only organs adjacent to the defect or relatively movable organs such as the small bowel and transverse colon were herniated. Two cases were accompanied by stomach herniation with the volvulus and liver, respectively. The duration from presentation to diagnosis varied from 5 days to 1 year. Diagnoses were made by chest X-ray, upper gastrointestinal series and chest computed tomography. All patients underwent primary repair with interrupted non-absorbable sutures by a transabdominal approach. None had postoperative complications. The follow-up period in six patients ranged from 4 months to 20 years (median 3.8 years). There was no recurrence in any of the patients on follow-up.
Late-presenting congenital diaphragmatic hernia (CDH) has been defined as CDH diagnosed after the neonatal period due to initial symptoms after the neonatal period or asymptomatic CDH found in the course of routine X-ray examination of the chest beyond the neonatal period.1,2 The incidence of late-presenting CDH has been quoted to be as high as 5-45.5% of all cases of CDH.3,4 Neonatal CDH is a well-recognized entity, but its presentations beyond the neonatal period varies, leading to clinical and radiological misdiagnoses. In contrast to the high mortality and morbidity rates for neonatal CDH, the prognosis for late-presenting CDH if diagnosed earlier is usually favorable. We retrospectively reviewed our experience with late-presenting CDH at a single institution to determine the characteristics of late-presenting CDH for early diagnosis.
After Institutional Review Board approval (SC12EISI0131), the medical records of children less than 16 years of age in whom a Bochdalek or Morgagni CDH were diagnosed after 30 days of age and received a operation by two surgeons in the single institution, between January 1981 and June 2012, were reviewed retrospectively. Patients with posttraumatic and hiatus hernias and eventration of the diaphragm were excluded. In our institution, intensive care unit stay and postoperative ventilator care were applied depending on the patient's conditions. Each child was evaluated with respect to age, sex, hernia site, presenting signs and symptoms, diagnostic method, operative findings, associated anomalies, postoperative morbidity and mortality, and long-term follow-up.
The incidence of late-presenting congenital diaphragmatic hernia in our institution was 18.9% (7 cases/total 37 cases). The patients' ages ranged from 2.5 months to 16 years. There were six boys and one girl. Five hernias were left-sided, one was right-sided, and one was a retrosternal hernia. Among the left-sided hernias, four had defects in the posterolateral side and one had a defect in the mid portion of the left diaphragm. The right-sided hernia had a defect in the posterolateral side. The defect sizes varied from 1.5×1.5 cm to 10×10 cm. The extra-abdominal associated anomalies included congenital heart disease in two cases and cerebral palsy in one case. All patients had normal intestinal rotation but case 4 had an abnormal ascending colon fixation (Table 1).
Non-specific symptoms and signs were presented. Three cases presented with gastrointestinal symptoms and signs such as vomiting or left upper quadrant (LUQ) pain initially and then respiratory symptoms such as dyspnea and cough (cases 3, 4, and 6).
In case 3, sudden vomiting and fever developed 10 days after visiting our hospital and cough and dyspnea started 3 days later. Although treated at a local clinics, the patient's condition did not improve. The diagnosis of CDH was made by chest X-ray in our hospital. In the operative findings, almost the entire small bowel and a large amount of the transverse colon including the splenic flexure were herniated through the posterolateral defect of the left diaphragm.
Case 4 had upper respiratory infection symptoms such as cough and rhinorrhea for 1 week with vomiting that developed at the same time for the initial 2 days. He was treated in a local clinic without improvement, and dyspnea developed 2 days after the visit to our hospital. In our emergency room, the patient had an endotracheal tube inserted due to cyanosis. The diagnosis of a right diaphragmatic hernia was made by chest X-ray. In the medical history, the patient had an operation because of a left inguinal hernia. Chest X-ray was normal at that time. In the operative findings, almost the entire small bowel and ascending colon without retroperitoneal fixation and the hepatic flexure of the transverse colon were herniated through the posterolateral defect of the right diaphragm.
Case 6 had intermittent mild abdominal pain for 1 month, followed by acute abdominal pain that developed on admission day. He was referred to our hospital based on the impression of an abdominal mass via chest X-ray (Fig. 1). In the abdominal CT, a left diaphragmatic hernia was confirmed. The organs herniated into the thoracic cavity included the stomach with organoaxial volvulus, colon, small bowel and part of spleen (Fig. 2). During the operation, we found herniation of the entire stomach, some of the transverse colon and small bowel and a part of the spleen through the posterolateral defect of the left diaphragm. However, we could not perform a manual reduction of the stomach because the stomach was too distended due to the gastric contents. Thus, we performed the reduction after the gastrostomy and decompression.
Two cases presented only gastrointestinal symptoms (cases 2 and 7). Case 2 had projectile and bile-stained vomiting, fever and poor oral intake for 5 days. Abdominal distension was found on physical examination. In the chest X-ray, left CDH was diagnosed. At the laparotomy, a large amount of ascites had accumulated, and the diffuse dilatation of the small bowel and colon was observed. Splenic flexure of the transverse colon was herniated through the 1.5-cm-sized defect in the center of the focal eventration in the mid portion of the left diaphragm. Case 7 had intermittent abdominal pain for 1 year. LUQ pain had developed 10 days before visiting our hospital. Case 7's vital signs were stable, and the diagnosis of left CDH was made by chest X-ray and an upper gastrointestinal series (UGIS). At the laparotomy, some segments of the small bowel and splenic flexure of the transverse colon were herniated through the posterolateral defect of the left diaphragm.
One case presented with only respiratory symptoms (case 1). Five days after a visit to our hospital, she had sudden cyanosis and, dyspnea for 5 min after a feeding. In the chest PA, the first impression was aspiration pneumonia. After 2 days of conservative treatment, cyanosis developed again. Thus, a chest CT was performed and left CDH was confirmed. During the operation, we found herniation of some segments of small bowel and splenic flexure of the transverse colon.
Case 5 presented with chronic upper respiratory symptoms with intermittent vomiting. CDH was suggested during the evaluation of congenital heart disease by chest X-ray. Through the UGIS, a Morgagni hernia was confirmed. In the operative findings, the left lateral lobe of the liver and a considerable segment of the transverse colon were herniated through the retrosternal defect.
The duration from presentation to diagnosis varied from 5 days to 1 year. In 2 cases, intermittent LUQ pain presented for 1 month and 1 year, followed by acute abdominal pain aggravated at 1 day and 10 days before the hospital visits, respectively (Table 1).
All patients underwent primary repair with interrupted non-absorbable sutures by a trans-abdominal approach. Case 1, 2, 4 and 6 received postoperative care in the intensive care unit for 1 day, 1 day, 4 days, and 2 days, respectively. Only one patient (case 4) received postoperative ventilator care for 20 hours (Table 1). No patients had postoperative complications. They were discharged from the 7th to the 13th postoperative day.
The follow-up period in these 6 patients ranged from 4 months to 20 years (median 3.8 years). There were no other postoperative complications or recurrence in any of the patients on follow-up.
CDH is the result of incomplete closure of the normal pleuroperitoneal canal during fetal development. Most cases are diagnosed prenatally or in the neonatal period. However, 5-45.5% of CDH may appear healthy during the newborn period, but abnormality can manifest in later life.3,4 The congenital defect is identical in newborns and older patients, but the symptoms, operative management and complications of CDH in the older patients differ considerably from those found in the new-born patients. In the literature, the male-to-female ratio was approximately 2 : 1,4 and in our study, the male-to-female ratio was 6 : 1. Infants with congenital diaphragmatic hernia is classified by the herniation time, the severity of pulmonary hypoplasia, survival, and the presence of late pulmonary sequelae.2 In the classification, type 1 refers to cases in which the herniation has occurred in the early period of rapid bronchial division. It results in the bilateral pulmonary hypoplasia and perinatal death. Type 2 refers to cases in which the herniation has occurred in the late period of rapid bronchial division. It results in unilateral pulmonary hypoplasia. Neonatal outcome of this disease is characterized by pulmonary hypoplasia, potential pulmonary hypertension and alterations of blood-gas. Moreover preoperative stabilization should be a priority in CDH treatment and stabilization should be guided by precise cardiorespiratory criteria.5,6 Some of these cases die in the neonatal period, whereas, others survive with considerable pulmonary problems. Type 3 refers to cases in which the herniation has occurred during either late gestation or the early neonatal period. Herniation might present at birth with mild respiratory distress. Pulmonary hypoplasia is minimal or absent in these patients and most of them survive with no detectable respiratory problems. In type 4, the herniation has presented after the neonatal period. They have no pulmonary hypoplasia, and survive with no respiratory problems.
In our opinion, type 3 could be divided into two groups: the early-presenting group and late-presenting group (those having symptoms beyond neonatal period). The latter group may have a small amount of abdominal viscera herniated into the thoracic cavity at late gestation or birth, which escapes detection because of the absence of symptoms. In addition, type 4 patients may have a defect with no herniation until an episode of increased intra-abdominal pressure causes the herniation.
Therefore, most late-presenting CDH belongs to late-presenting type 3 and type 4. The secondary abnormalities representing the effects of herniated abdominal viscera in the thorax are nonfixation of the mesentery with malrotation of the intestinal tract and lung hypoplasia.7 Therefore, late-presenting type 3 group may have intestinal malrotation and pulmonary hypoplasia, whereas the type 4 group does not have any intestinal malrotation and pulmonary hypoplasia. However, it is difficult to distinguish between late-presenting type 3 without malrotation or lung hypoplasia and type 4 if patients do not have a fortuitous chest radiograph demonstrating an apparently normal diaphragm during the neonatal period.
Associated anomalies in late-presenting CDH patients have been reported with variable frequencies, ranging from 8.6 to 80%.3,4,7-9 Hosgor, et al.7 reported a considerable incidence of 80% with a wide spectrum of associated malformations. Malrotation and pulmonary hypoplasia were mentioned as the two most common secondary malformations. Pulmonary hypoplasia and the cardiac anomalies was associated with the postoperative morbidity and the preoperative mortality. We guess that most cases with malrotation and pulmonary hypoplasia in this study may belong to the late-presenting type 3 group. In our study, however, none of the patients had intestinal malrotation or pulmonary hypoplasia. Only three cases (23%) had associated anomalies, such as cardiac anomalies (2 cases) or cerebral palsy and incomplete fixation (1 case). Additionally, none had postoperative complications. Moreover, although six cases were not checked with a chest X-ray before the presentation, one case had a normal chest X-ray before the presentation of CDH. We presume that our cases may belong to late-presenting type 3 without malrotation or pulmonary hypoplasia and type 4.
The contents of the hernia are associated with the defect site. In the literature, the large bowel, small bowel, stomach, and spleen were herniated successively in left CDH. The liver, small bowel, and large bowel were herniated in order in right CDH.3 In our cases, because intestinal malrotations were absent, only organs adjacent to the defect or relatively movable organs, such as the small bowel and transverse colon were herniated. Two cases were accompanied by herniation of the stomach and liver, respectively.
Diagnosis is frequently made by chest X-ray, but it is not always diagnostic because the chest X-ray may mimic lower lobe pneumonia, diaphragmatic eventration, pneumothorax, pleural effusion, and diaphragmatic mass, thereby potentially leading to a misdiagnosis.9-12 As a result, such a misdiagnosis together with a non-specific clinical presentation can delay the diagnosis. In these cases, contrast studies of the gastrointestinal tract or especially a chest or abdominal CT can help with diagnosis.10
Once diagnosed, early surgical intervention is necessary for the prevention of complications. Patients with late-presenting CDH have a more favorable prognosis due to less severe or absent lung hypoplasia.13 Acute gastric volvulus associated with CDH is extremely rare but can have life-threatening complications. It is associated with the elongation or absence of 2 of the 4 ligaments of the stomach with the connection to the left diaphragm. There are three types of Gastric volvulus such as organoaxial, mesenteroaxial, or combined. Increased intraabdominal pressure can lead to fatal results, such as gastric strangulation, ischaemia, perforation, pancreatitis, peritonitis, shock, and death with the mortality of 80%.14-19
Late-presenting CDH with gastric volvulus is usually misdiagnosed as a tension pneumothorax or pneumonia with cavitation, pleural effusion and pneumatoceles.10,14-16 This common error, unfortunately, often results in iatrogenic gastric perforation due to the placement of an unnecessary chest tube. In these patients, initial plain chest films show a large sign bubble with an air-fluid level in the left chest. One report emphasized that the abnormal position of the gastric bubble or absence of the gastric bubble in the abdomen, makes the early diagnosis of late-presenting CDH on the left side.10 If CHD is suggested, it can be diagnosed easily by the chest radiograph with a nasogastric tube insertion, when we look for the placement of the tube in the thoracic cavity. If not able to pass an NG tube, chest CT and magnetic resonance scans are helpful. Acute gastric volvulus is a surgical emergency. Thus, surgery should not be delayed for further imaging studies once the diagnosis is made from the chest plain X-ray. Often, a herniated stomach, especially in cases involving volvulus, is too distended. In these cases, needle or open decompression of the stomach can help reduce the distension of the stomach into the abdomen.
In conclusion, late-presenting CDH that can be classified as late-presenting type 3 or type 4 CDH can have a wide spectrum of clinical manifestations, and a high index of suspicion is important for diagnosis because it can become a life-threatening condition, such as CDH with gastric volvulus. Early diagnosis and appropriate treatment can lead to a good prognosis.
Figures and Tables
Fig. 1
Preoperative chest radiographs (A: posteroanterior, B: lateral) in case 6 showing a large gastric air fluid level and air within the bowel loops herniated into the left chest.
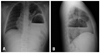
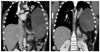
Fig. 2
Chest CT scan in case 6 showing a herniated stomach with huge distension passing a NG tube (A) and organoaxial torsion of the stomach (B). NG, nasogastric.
References
2. Wiseman NE, MacPherson RI. "Acquired" congenital diaphragmatic hernia. J Pediatr Surg. 1977; 12:657–665.

3. Elhalaby EA, Abo Sikeena MH. Delayed presentation of congenital diaphragmatic hernia. Pediatr Surg Int. 2002; 18:480–485.

4. Bagłaj M. Late-presenting congenital diaphragmatic hernia in children: a clinical spectrum. Pediatr Surg Int. 2004; 20:658–669.

5. Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology. 2010; 98:354–364.

6. Gentili A, Giuntoli L, Bacchi Reggiani ML, Masciopinto F, Lima M, Baroncini S. Neonatal congenital diaphragmatic hernia: respiratory and blood-gas derived indices in choosing surgical timing. Minerva Anestesiol. 2012; 78:1117–1125.
7. Hosgor M, Karaca I, Karkiner A, Ucan B, Temir G, Erdag G, et al. Associated malformations in delayed presentation of congenital diaphragmatic hernia. J Pediatr Surg. 2004; 39:1073–1076.

8. Chang SW, Lee HC, Yeung CY, Chan WT, Hsu CH, Kao HA, et al. A twenty-year review of early and late-presenting congenital Bochdalek diaphragmatic hernia: are they different clinical spectra? Pediatr Neonatol. 2010; 51:26–30.

9. Baerg J, Kanthimathinathan V, Gollin G. Late-presenting congenital diaphragmatic hernia: diagnostic pitfalls and outcome. Hernia. 2012; 16:461–466.

10. Muzzafar S, Swischuk LE, Jadhav SP. Radiographic findings in late-presenting congenital diaphragmatic hernia: helpful imaging findings. Pediatr Radiol. 2012; 42:337–342.

11. Waseem M, Quee F. A wheezing child: breath sounds or bowel sounds? Pediatr Emerg Care. 2008; 24:304–306.
12. Strunk T, Simmer K, Kikiros C, Patole S. Late-onset right-sided diaphragmatic hernia in neonates-case report and review of the literature. Eur J Pediatr. 2007; 166:521–526.

13. Weber TR, Tracy T Jr, Bailey PV, Lewis JE, Westfall S. Congenital diaphragmatic hernia beyond infancy. Am J Surg. 1991; 162:643–646.

14. Ayala JA, Naik-Mathuria B, Olutoye OO. Delayed presentation of congenital diaphragmatic hernia manifesting as combined-type acute gastric volvulus: a case report and review of the literature. J Pediatr Surg. 2008; 43:E35–E39.

15. Vandy FC, Landrum JE, Gerig NR, Prahlow JA. Death due to late-presenting congenital diaphragmatic hernia in a 2-year-old child. Am J Forensic Med Pathol. 2008; 29:75–79.

17. Schimpl G, Fotter R, Sauer H. Congenital diaphragmatic hernia presenting after the newborn period. Eur J Pediatr. 1993; 152:765–768.

18. Sharma S, Gopal SC. Gastric volvulus with perforation in association with congenital diaphragmatic hernia. Indian J Pediatr. 2004; 71:948.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download