Abstract
Purpose
For the successful completion of transcatheter closure of atrial septal defects with the Amplatzer septal occluder, shape of the defects should be considered prior to selecting the device. The purpose of this study is to evaluate the results of a transcatheter closure of oval shaped atrial septal defect.
Materials and Methods
From November 2009 until November 2011, cardiac computed tomography was performed on 69 patients who needed a transcatheter closure of atrial septal defect. We defined an oval shaped atrial septal defect as the ratio of the shortest diameter to the longest diameter ≤0.75 measured using computed tomography. A trans-thoracic echocardiogram was performed one day after and six months after.
Results
The transcatheter closure of atrial septal defect was performed successfully in 24 patients in the ovoid group and 45 patients in the circular group. There were no serious complications in both groups and the complete closure rate at 6 months later was 92.3% in the ovoid group and 93.1% in the circular group (p>0.05). The differences between the device size to the longest diameter of the defect and the ratios of the device size to the longest diameter were significantly smaller in the ovoid group (1.8±2.8 vs. 3.7±2.6 and 1.1±0.1 vs. 1.2±0.2).
Atrial septal defect (ASD) is one of the most common congenital heart diseases, with an incidence of approximately 1 per 100 live births, based on a previous report.1 Surgical closure was considered once as the best treatment.2,3 However, device closure with the Amplatzer septal occluder (ASO) (AGA medical, Golden Valley, MN, USA) has gradually become a competitive alternative.4,5 For successful device closure of ASD, an accurate size evaluation of the defect, as well as a detailed morphology description of the defect, is essential. In general, ASD provides various shapes including circular, oval and complex morphology.6,7 Even though good long-term results of a transcatheter closure of ASD with ASO have been reported,8,9 it is difficult to find a specific report regarding the transcatheter closure of ASD, based on various morphologies. We realize that many ASDs are not circular, but oval in developed cardiac tomographic images. When using device closure for non-circular ASD, the size of device should be considered according to the various diameters of the defect. One diameter of the circular device could be too large for the shortest diameter causing a deformity in residual rims. The cardiac erosion after successful implantation as one of the most important complications is supposed to occur due to a larger device in the oval defect compared to the circular defect. Therefore, the purpose of the present study is to evaluate the outcome of a transcatheter closure of oval shaped ASD.
This is a retrospective chart review of patients undergoing transcatheter closure of ASD. The inclusion criteria were all patients with secundum ASD deemed appropriate for transcatheter closure with ASO. In addition, a cardiac computed tomography was checked before the transcatheter closure of ASD from November 2009 until November 2011 at Sejong General Hospital in South Korea. Four patients were excluded and among them two patients had 2 or more defects, one patient had an extremely complex shape that was overlapped with 2 circular defects, and one patient had apolygonal shape upon computed tomography. In total, 69 patients were included and retrospectively reviewed for medical records. For the decision of the transcatheter closure of ASD with ASO, a trans-thoracic echocardiogram was first performed. If the defect was not definite, due to the poor window in the trans-thoracic echocardiogram, trans-esophageal echocardiogram was done in selected patients. The inclusion criteria for the transcatheter closure of ASD with ASO were as follows: 2' ASD with the evidence of right ventricular volume overload, no significant pulmonary arterial hypertension that was irreversible, no significant arrhythmia, no serious problems in other organs and presumptive appropriate rims for ASO implantation. Cardiac computed tomography was performed. If necessary, oral or intravenous beta-blockers were used. One operator under the guidance of an intra-cardiac echocardiogram performed the device closure. We decided the device size based on the measured data from various echocardiographic images and images from cardiac computed tomography. Usually, the longest two-dimensional diameter, among all of the images from trans-thoracic echocardiogram, cardiac computed tomography and intra-cardiac echocardiogram, was considered for the decision of the device size. From the en face view in the computed tomography images, the longest diameter and the shortest diameter were measured (Fig. 1). All diameters of the defect were measured at the end-systolic phase. We typically chose a device that is 0-4 mm larger than the longest diameter of the defect at the first trial, but this depends on the flexibility of the adjacent septal rims. Further, if successful implantation failed or oversizing was suggested, we changed the size of the device. Before deployment of the device, we pushed the cable and made the device push toward the left atrium for one second, and then we confirmed the stability of the device. We referred to this as a modified Minnesota wiggles. We defined the ovoid group with oval shaped septal defect as the ratio of the shortest diameter to the longest diameter (b/a) below 0.75, while the circular group had circular shaped septal defect if the ratio of the shortest diameter to the longest diameter was over 0.75.6 The differences between the longest diameter of the defect and the device size were calculated in the two groups. In addition, the ratios of the device size to the longest diameter of the defect were compared between the two groups. The residual shunt and any complications were checked from a follow-up trans-thoracic echocardiogram on the following day and 6 months later.
Data are expressed as the mean±standard deviation. To compare the results of both groups, a nonparametric Mann-Whitney test and a Fisher's exact test were performed using SPSS 11.5 (SPSS Inc., Chicago, IL, USA) due to our small sample size. p-values <0.05 were considered statistically significant.
The locally appointed ethics committee approved this research protocol.
In the computed tomography image of all patients, the longest diameter (a) and the shortest diameter (b) were 21.4±7.6 mm (8.0-45.0 mm) and 16.7±5.6 mm (4.0-31.0 mm), respectively. The ratio of the shortest diameter to the longest diameter (b/a) was 0.79±0.11 (0.5-1.0). Only three patients had ratios of 1.0, and ten patients had ratios over 0.9. Of the total enrolled patients, 24 patients (34.8%) were assigned in the ovoid group and 45 patients (65.2%) in the circular group (Table 1).
In the ovoid group, data from 6 males and 18 females were reviewed. The mean age was 36.2±16.6 (15-77) years, and the mean body weight was 59.0±12.6 (38-99) kg. From the computed tomography images, the longest diameter (a) was 23.7±8.6 mm, and the ratio (b/a) was 0.67±0.07 (0.5-0.75). From the catheterization results, the shunt ratio (Qp/Qs) was 3.2±1.7. In contrast, the circular group consisted of 14 males and 31 females. The mean age was 42.1±12.7 years and the mean body weight was 60.9±10.9 kg. The longest diameter (a) and the ratio (b/a) were 20.2±6.8 mm and 0.85±0.06, respectively. The shunt ratio (Qp/Qs) in the circular group was 2.8±1.2. When we compared the two groups, the body weights, the shunt ratios (Qp/Qs), and the longest diameters (a) were not statistically different, but the mean age of the ovoid group was younger than of the circular group (Table 1).
In the total population, the differences between the longest diameter of the defect (a) and the device size were 3.0±2.8 (range -7.0 to 11.0) mm. In addition, the ratios of the device size to the longest diameter of the defect were 1.19±0.20 (range 0.84 to 1.93).
A comparison between the two groups indicated that the differences between the longest diameter of the defect and the device size of the ovoid group were significantly smaller than the circular group (1.8±2.8 mm vs. 3.7±2.6 mm, p=0.009). Further, the ratios of the device size to the longest diameter of the defect in the ovoid group were also significantly smaller than those in the circular group (1.1±0.1 vs. 1.2±0.2, p=0.02) (Table 2).
Especially for the three patients in the ovoid group, the differences between the longest diameter of the defect and the device size showed negative numbers, -1, -1 and -7, which meant that the longest diameters of the defect were larger than the waist diameters of the device. In addition, the ratio of the shortest diameter to the longest diameter (b/a) were 0.72, 0.67 and 0.53, respectively (Table 3). In the last case that showed -7, the longest diameter of the defect was 45 mm and the shortest diameter of the defect was 24 mm (b/a=0.53). For this oval defect, ASO of 38 mm was implanted successfully, and complete closure was confirmed by trans-thoracic echocardiogram immediately and 6 months later even though the immediate cardiac computed tomography image showed a small gap from the waist of the device to an anterior margin of the defect (Fig. 2). On the other hand, the minimum level of the differences between the longest diameter of the defect and the device size in the circular group was 0 mm.
During the procedure, no significant complications occurred. The follow-up results showed no embolization, no pericardial effusion, no newly developed arrhythmia, nor mitral regurgitation in both groups. Immediate trans-thoracic echocardiogram showed complete closure in 17 of the 24 patients in the ovoid group (70.8%), and follow-up trans-thoracic echocardiogram performed 6 months later showed complete closure in 12 of the 13 patients in the ovoid group (92.3%). At the same time, the immediate complete closure rate of the circular group was 80.0% (36/45) and at 6 months follow-up was 93.1% (27/29). However, there were no significant differences between the two groups (p>0.05) (Fig. 3).
From our study, we could observe that there was a higher incidence of ASD in females than male and completely circular ASD (b/a=1.0) was rare (n=3).
For a successful device closure of ASD, a trans-esophageal echocardiogram can assess the size, anatomy, and suitability of the lesion for closure. A two-dimensional echocardiogram can integrate multiple image planes for the operator to reconstruct a three-dimensional anatomy of ASD, but has limitations of the visualization of an accurate shape of the defect. However, an accurate dimension related to the shape of the defect is considered important for the successful transcatheter closure because the ASO is uniform. There are studies that have described the complex shapes of ASD with a three-dimensional trans-esophageal echocardiogram.6,7,9,10 However, we did not perform a three-dimensional trans-esophageal echocardiogram for our patients' series. Trans-esophageal echocardiogram requires sedation and can potentially damage the esophagus. Cardiac computed tomography can be considered an alternative because Ko, et al.11 reported that cardiac computed tomography is very helpful in the noninvasive evaluation for ASO implantation of ASD. Nevertheless, general concerns about cardiac computed tomography is the exposure to harmful radiation. We did not check the radiation dose for all the patients, but cardiac computed tomography was performed with minimal radiation. We performed cardiac computed tomography with 0.2-0.6 mSv for small children and 1.7-1.9 mSv for adults. This minimal exposure to radiation helped us evaluate ASD size and morphology by cardiac computed tomography.
When we defined oval shaped ASD as the shortest diameter ≤75% of the longest diameter measured from en face image in cardiac computed tomography, oval shaped ASD formed 34.8% of our total patients. Johri, et al.6 reported 42% of oval and 33% of complex ASD shapes in 25 ASDs using a real-time three-dimensional echocardiogram. We realize that many ASDs are not circular in shape.
Regarding the device size, balloon sizing has been considered an integral part of transcatheter closure of ASD with ASO. However, there have been several experiences without balloon sizing.12,13 In one particular case of oval shaped ASD, the effect of a balloon inflation would alter the shape of the defect to conform to the relatively circular shape of the balloon. Moreover, based on Zanchetta's study,14 the device size in the oval shaped ASD could be smaller than the longest diameter by intra-cardiac echocardiogram measurement: d=√(a×b). Although we did not apply this formula, we had three cases whose differences between the device size and the longest diameter of the defect were below zero, and all of them were in the ovoid group. In one patient whose ratio (b/a) was 0.53 and the longest diameter of the defect was 45 mm, we chose an ASO of 38 mm because it was the maximum size of ASO available in our country at that time. This defect was closed successfully. The waist diameter of the device was smaller than the longest diameter of the defect, yet the entire length of the device was much larger than 45 mm and no residual leak was detected by trans-thoracic echocardiogram immediately as well as 6 months later.
It is recommended that oversizing should be avoided due to the risks of mushrooming deformity, impingement on cardiovascular structures, and other serious complications.15,16 Our data showed successful device closure with ASO for oval shaped ASD. In another study, the residual shunt at one day after device closure was found in 8.6% and the residual shunt at three months later was found only in 1.3% of patients.4 The complete closure rates in our study were a little higher, but we could expect nearly the same results afterward. There were no differences in the occurrence of complications, immediate, and mid-term complete closure rate. Interestingly, the mean upsizing of the device in the ovoid group was significantly lower than in the circular group, which matched with Zanchetta's concept.14 Our results showed that the device diameter to be 1.8 mm longer than the longest diameter of defect in ovoid group, whereas a device 3.7 mm longer was used for the circular group. However, this depends on the ratio (b/a) of the defect.
One limitation of our study is that it is not a randomized control study. This retrospective analysis was based on patients who underwent a transcatheter closure of ASD with ASO, performed by experienced operators. In our study, the evaluation of the defect size and shape was based on cardiac computed tomography exclusively so the patients without pre-interventional cardiac computed tomography were excluded. Small children were excluded because of the difficulties in cardiac computed tomography; therefore, there could be some selection bias in our study. However, the pre-interventional cardiac computed tomography was applied randomly.
Although not significant, the longest diameters of the defects in the ovoid group were bigger than those were in the circular group. This could be another reason for the relatively smaller ratio of the device chosen for the closure of defects in the ovoid group. The biggest limitation might be that the device size, chosen by these operators, was not based on a constant equation, but on a personal experience. Further, it would be better if we had data on device closure of oval shaped ASD with a balloon sizing method. A formula applicable for the proper selection of the device for non-circular ASD is needed. Moreover, our computed tomography system, which provides low dose radiation, is very beneficial; however, we are not sure if the computed tomography is superior to a three dimensional echocardiogram.
In conclusion, transcatheter closure of ASD with ASO was found safe and effective even for oval shaped ASD. Oval shaped ASD can be successfully closed with a smaller size of ASO compared to circular ASD.
Figures and Tables
Fig. 1
The reconstructed en face image from cardiac computed tomography shows the longest diameter (a) and the shortest diameter (b).

Fig. 2
(A) The tomographic image shows oval shaped atrial septal defect (arrows). (B) There is a small gap between the waist of the device and the anterior margin of the defect on computed tomographic image of patient C immediately after implantation.
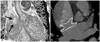
Fig. 3
The complete closure rates the next day and 6 months after were not statistically different in both groups (p>0.05).

Table 1
Characteristics of the Patients with Oval Shaped Atrial Septal Defect (Ovoid Group) and Circular Atrial Septal Defect (Circular Group) Show No Significant Difference Except Age

References
1. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002; 39:1890–1900.

2. Barratt-Boyes BG, Kirklin JW. Cardiac Surgery. New York: Churchill Livingstone;1993. p. 609–644.
3. Galal MO, Wobst A, Halees Z, Hatle L, Schmaltz AA, Khougeer F, et al. Peri-operative complications following surgical closure of atrial septal defect type II in 232 patients--a baseline study. Eur Heart J. 1994; 15:1381–1384.

4. Masura J, Gavora P, Podnar T. Long-term outcome of transcatheter secundum-type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005; 45:505–507.

5. Yew G, Wilson NJ. Transcatheter atrial septal defect closure with the Amplatzer septal occluder: five-year follow-up. Catheter Cardiovasc Interv. 2005; 64:193–196.

6. Johri AM, Witzke C, Solis J, Palacios IF, Inglessis I, Picard MH, et al. Real-time three-dimensional transesophageal echocardiography in patients with secundum atrial septal defects: outcomes following transcatheter closure. J Am Soc Echocardiogr. 2011; 24:431–437.

7. Roberson DA, Cui W, Patel D, Tsang W, Sugeng L, Weinert L, et al. Three-dimensional transesophageal echocardiography of atrial septal defect: a qualitative and quantitative anatomic study. J Am Soc Echocardiogr. 2011; 24:600–610.

8. Knepp MD, Rocchini AP, Lloyd TR, Aiyagari RM. Long-term follow up of secundum atrial septal defect closure with the amplatzer septal occluder. Congenit Heart Dis. 2010; 5:32–37.

9. Acar P. Three-dimensional echocardiography in transcatheter closure of atrial septal defects. Cardiol Young. 2000; 10:484–492.

10. Huang X, Shen J, Huang Y, Zheng Z, Fei H, Hou Y, et al. En face view of atrial septal defect by two-dimensional transthoracic echocardiography: comparison to real-time three-dimensional transesophageal echocardiography. J Am Soc Echocardiogr. 2010; 23:714–721.

11. Ko SF, Liang CD, Yip HK, Huang CC, Ng SH, Huang CF, et al. Amplatzer septal occluder closure of atrial septal defect: evaluation of transthoracic echocardiography, cardiac CT, and transesophageal echocardiography. AJR Am J Roentgenol. 2009; 193:1522–1529.

12. Zanchetta M, Onorato E, Rigatelli G, Pedon L, Zennaro M, Carrozza A, et al. Intracardiac echocardiography-guided transcatheter closure of secundum atrial septal defect: a new efficient device selection method. J Am Coll Cardiol. 2003; 42:1677–1682.

13. Gupta SK, Sivasankaran S, Bijulal S, Tharakan JM, Harikrishnan S, Ajit K. Trans-catheter closure of atrial septal defect: Balloon sizing or no Balloon sizing-single centre experience. Ann Pediatr Cardiol. 2011; 4:28–33.

14. Zanchetta M. On-line intracardiac echocardiography alone for Amplatzer Septal Occluder selection and device deployment in adult patients with atrial septal defect. Int J Cardiol. 2004; 95:61–68.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download